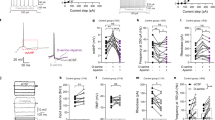
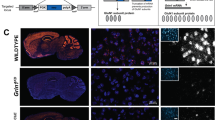
Abstract
NMDA glutamate receptors have key roles in brain development, function and dysfunction. Regulatory roles of D-serine in NMDA receptor-mediated synaptic plasticity have been reported. Nonetheless, it is unclear whether and how neonatal deficits in NMDA-receptor-mediated neurotransmission affect adult brain functions and behavior. Likewise, the role of D-serine during development remains elusive. Here we report behavioral and electrophysiological deficits associated with the frontal cortex in Pick1 knockout mice, which show D-serine deficits in a neonatal- and forebrain-specific manner. The pathological manifestations observed in adult Pick1 mice are rescued by transient neonatal supplementation of D-serine, but not by a similar treatment in adulthood. These results indicate a role for D-serine in neurodevelopment and provide novel insights on how we interpret data of psychiatric genetics, indicating the involvement of genes associated with D-serine synthesis and degradation, as well as how we consider animal models with neonatal application of NMDA receptor antagonists.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Paré D . Presynaptic induction and expression of NMDA-dependent LTP. Trends Neurosci 2004; 27: 440–441.
Anwyl R . Induction and expression mechanisms of postsynaptic NMDA receptor-independent homosynaptic long-term depression. Prog Neurobiol 2006; 78: 17–37.
Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA et al. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 2006; 125: 775–784.
Snyder SH, Kim PM . D-amino acids as putative neurotransmitters: focus on D-serine. Neurochem Res 2000; 25: 553–560.
Wolosker H . D-serine regulation of NMDA receptor activity. Sci STKE 2006; 2006: pe41.
Martineau M, Baux G, Mothet JP . D-serine signalling in the brain: friend and foe. Trends Neurosci 2006; 29: 481–491.
Lee JM, Zipfel GJ, Choi DW . The changing landscape of ischaemic brain injury mechanisms. Nature 1999; 399: A7–A14.
Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci 2005; 8: 1051–1058.
Milnerwood AJ, Raymond LA . Early synaptic pathophysiology in neurodegeneration: insights from Huntington's disease. Trends Neurosci 2010; 33: 513–523.
Kalia LV, Kalia SK, Salter MW . NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol 2008; 7: 742–755.
Swanson RA, Ying W, Kauppinen TM . Astrocyte influences on ischemic neuronal death. Curr Mol Med 2004; 4: 193–205.
Rossi DJ, Brady JD, Mohr C . Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci 2007; 10: 1377–1386.
Sasabe J, Chiba T, Yamada M, Okamoto K, Nishimoto I, Matsuoka M et al. D-serine is a key determinant of glutamate toxicity in amyotrophic lateral sclerosis. EMBO J 2007; 26: 4149–4159.
Ben-Ari Y . Developing networks play a similar melody. Trends Neurosci 2001; 24: 353–360.
Blankenship AG, Feller MB . Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci 2010; 11: 18–29.
Leinekugel X, Medina I, Khalilov I, Ben-Ari Y, Khazipov R . Ca2+ oscillations mediated by the synergistic excitatory actions of GABA(A) and NMDA receptors in the neonatal hippocampus. Neuron 1997; 18: 243–255.
Allène C, Cattani A, Ackman JB, Bonifazi P, Aniksztejn L, Ben-Ari Y et al. Sequential generation of two distinct synapse-driven network patterns in developing neocortex. J Neurosci 2008; 28: 12851–12863.
Voigt T, Opitz T, de Lima AD . Activation of early silent synapses by spontaneous synchronous network activity limits the range of neocortical connections. J Neurosci 2005; 25: 4605–4615.
Fujii K, Maeda K, Hikida T, Mustafa AK, Balkissoon R, Xia J et al. Serine racemase binds to PICK1: potential relevance to schizophrenia. Mol Psychiatry 2006; 11: 150–157.
Focant MC, Goursaud S, Boucherie C, Dumont AO, Hermans E . PICK1 expression in reactive astrocytes within the spinal cord of amyotrophic lateral sclerosis (ALS) rats. Neuropathol Appl Neurobiol 2013; 39: 231–242.
Hikida T, Mustafa AK, Maeda K, Fujii K, Barrow RK, Saleh M et al. Modulation of D-serine levels in brains of mice lacking PICK1. Biol Psychiatry 2008; 63: 997–1000.
Gardner SM, Takamiya K, Xia J, Suh JG, Johnson R, Yu S et al. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron 2005; 45: 903–915.
Suh YH, Pelkey KA, Lavezzari G, Roche PA, Huganir RL, McBain CJ et al. Corequirement of PICK1 binding and PKC phosphorylation for stable surface expression of the metabotropic glutamate receptor mGluR7. Neuron 2008; 58: 736–748.
Terashima A, Pelkey KA, Rah JC, Suh YH, Roche KW, Collingridge GL et al. An essential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron 2008; 57: 872–882.
Steinberg JP, Takamiya K, Shen Y, Xia J, Rubio ME, Yu S et al. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron 2006; 49: 845–860.
Xiao N, Kam C, Shen C, Jin W, Wang J, Lee KM et al. PICK1 deficiency causes male infertility in mice by disrupting acrosome formation. J Clin Invest 2009; 119: 802–812.
Clem RL, Anggono V, Huganir RL . PICK1 regulates incorporation of calcium-permeable AMPA receptors during cortical synaptic strengthening. J Neurosci 2010; 30: 6360–6366.
Atianjoh FE, Yaster M, Zhao X, Takamiya K, Xia J, Gauda EB et al. Spinal cord protein interacting with C kinase 1 is required for the maintenance of complete Freund's adjuvant-induced inflammatory pain but not for incision-induced post-operative pain. Pain 2010; 151: 226–234.
Hu ZL, Huang C, Fu H, Jin Y, Wu WN, Xiong QJ et al. Disruption of PICK1 attenuates the function of ASICs and PKC regulation of ASICs. Am J Physiol Cell Physiol 2010; 299: C1355–C1362.
Wang W, Petralia RS, Takamiya K, Xia J, Li YQ, Huganir RL et al. Preserved acute pain and impaired neuropathic pain in mice lacking protein interacting with C Kinase 1. Mol Pain 2011; 7: 11.
Anggono V, Clem RL, Huganir RL . PICK1 loss of function occludes homeostatic synaptic scaling. J Neurosci 2011; 31: 2188–2196.
Volk L, Kim CH, Takamiya K, Yu Y, Huganir RL . Developmental regulation of protein interacting with C kinase 1 (PICK1) function in hippocampal synaptic plasticity and learning. Proc Natl Acad Sci USA 2010; 107: 21784–21789.
Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S et al. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci USA 2007; 104: 14501–14506.
Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H et al. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry 2008; 13: 115.
Ayhan Y, Abazyan B, Nomura J, Kim R, Ladenheim B, Krasnova IN et al. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Mol Psychiatry 2010; 16: 293–306.
Abazyan B, Nomura J, Kannan G, Ishizuka K, Tamashiro KL, Nucifora F et al. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol Psychiatry 2010; 68: 1172–1181.
Ibi D, Nagai T, Koike H, Kitahara Y, Mizoguchi H, Niwa M et al. Combined effect of neonatal immune activation and mutant DISC1 on phenotypic changes in adulthood. Behav Brain Res 2010; 206: 32–37.
Nagai T, Kitahara Y, Ibi D, Nabeshima T, Sawa A, Yamada K . Effects of antipsychotics on the behavioral deficits in human dominant-negative DISC1 transgenic mice with neonatal polyI:C treatment. Behav Brain Res 2011; 225: 305–310.
Niwa M, Kamiya A, Murai R, Kubo K, Gruber AJ, Tomita K et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron 2010; 65: 480–489.
Andreasson KI, Savonenko A, Vidensky S, Goellner JJ, Zhang Y, Shaffer A et al. Age-dependent cognitive deficits and neuronal apoptosis in cyclooxygenase-2 transgenic mice. J Neurosci 2001; 21: 8198–8209.
Tseng KY, O'Donnell P . Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci 2004; 24: 5131–5139.
Hashimoto K, Fujita Y, Horio M, Kunitachi S, Iyo M, Ferraris D et al. Co-administration of a D-amino acid oxidase inhibitor potentiates the efficacy of D-serine in attenuating prepulse inhibition deficits after administration of dizocilpine. Biol Psychiatry 2009; 65: 1103–1106.
Braff DL, Geyer MA, Swerdlow NR . Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001; 156: 234–258.
Wietrzych M, Meziane H, Sutter A, Ghyselinck N, Chapman PF, Chambon P et al. Working memory deficits in retinoid X receptor gamma-deficient mice. Learn Mem 2005; 12: 318–326.
Meyer-Lindenberg A, Weinberger DR . Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci 2006; 7: 818–827.
Ma TM, Abazyan S, Abazyan B, Nomura J, Yang C, Seshadri S et al. Pathogenic disruption of DISC1-serine racemase binding elicits schizophrenia-like behavior via D-serine depletion. Mol Psychiatry 2012; 18: 557–567.
Duffy S, Labrie V, Roder JC . D-serine augments NMDA-NR2B receptor-dependent hippocampal long-term depression and spatial reversal learning. Neuropsychopharmacology 2008; 33: 1004–1018.
Malkesman O, Austin DR, Tragon T, Wang G, Rompala G, Hamidi AB et al. Acute d-serine treatment produces antidepressant-like effects in rodents. Int J Neuropsychopharmacol 2012; 15: 1135–1148.
Bado P, Madeira C, Vargas-Lopes C, Moulin TC, Wasilewska-Sampaio AP, Maretti L et al. Effects of low-dose D-serine on recognition and working memory in mice. Psychopharmacology (Berl) 2011; 218: 461–470.
Javitt DC, Zukin SR . Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 1991; 148: 1301–1308.
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 1994; 51: 199–214.
Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R . Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry 1959; 81: 363–369.
Rosenbaum G, Cohen BD, Luby ED, Gottlieb JS, Yelen D . Comparison of sernyl with other drugs: simulation of schizophrenic performance with sernyl, LSD-25, and amobarbital (amytal) sodium; I. Attention, motor function, and proprioception. AMA Arch Gen Psychiatry 1959; 1: 651–656.
O'Donnell P . Adolescent onset of cortical disinhibition in schizophrenia: insights from animal models. Schizophr Bull 2011; 37: 484–492.
Sawa A . Cortical development and glutamatergic dysregulation in schizophrenia. Biol Psychiatry 2009; 66: 530–532.
Deutsch SI, Burket JA, Katz E . Does subtle disturbance of neuronal migration contribute to schizophrenia and other neurodevelopmental disorders? Potential genetic mechanisms with possible treatment implications. Eur Neuropsychopharmacol 2010; 20: 281–287.
Semba J, Tanaka N, Wakuta M, Suhara T . Neonatal phencyclidine treatment selectively attenuates mesolimbic dopamine function in adult rats as revealed by methamphetamine-induced behavior and c-fos mRNA expression in the brain. Synapse 2001; 40: 11–18.
Harich S, Gross G, Bespalov A . Stimulation of the metabotropic glutamate 2/3 receptor attenuates social novelty discrimination deficits induced by neonatal phencyclidine treatment. Psychopharmacology (Berl) 2007; 192: 511–519.
Nakatani-Pawlak A, Yamaguchi K, Tatsumi Y, Mizoguchi H, Yoneda Y . Neonatal phencyclidine treatment in mice induces behavioral, histological and neurochemical abnormalities in adulthood. Biol Pharm Bull 2009; 32: 1576–1583.
Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Han L et al. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry 2009; 14: 719–727.
Foltyn VN, Bendikov I, De Miranda J, Panizzutti R, Dumin E, Shleper M et al. Serine racemase modulates intracellular D-serine levels through an alpha,beta-elimination activity. J Biol Chem 2005; 280: 1754–1763.
Detera-Wadleigh SD, McMahon FJ . G72/G30 in schizophrenia and bipolar disorder: review and meta-analysis. Biol Psychiatry 2006; 60: 106–114.
Hayden EP, Nurnberger JI . Molecular genetics of bipolar disorder. Genes Brain Behav 2006; 5: 85–95.
Craddock N, O'Donovan MC, Owen MJ . Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull 2006; 32: 9–16.
Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014; 511: 421–427.
Bendikov I, Nadri C, Amar S, Panizzutti R, De Miranda J, Wolosker H et al. A CSF and postmortem brain study of D-serine metabolic parameters in schizophrenia. Schizophrenia Res 2007; 90: 41–51.
Hashimoto K, Engberg G, Shimizu E, Nordin C, Lindstrom LH, Iyo M . Reduced D-serine to total serine ratio in the cerebrospinal fluid of drug naive schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry 2005; 29: 767–769.
Brouwer A, Luykx JJ, van Boxmeer L, Bakker SC, Kahn RS . NMDA-receptor coagonists in serum, plasma, and cerebrospinal fluid of schizophrenia patients: a meta-analysis of case-control studies. Neurosci Biobehav Rev 2013; 37: 1587–1596.
Ozeki Y, Pickard BS, Kano S, Malloy MP, Zeledon M, Sun DQ et al. A novel balanced chromosomal translocation found in subjects with schizophrenia and schizotypal personality disorder: altered l-serine level associated with disruption of PSAT1 gene expression. Neurosci Res 2011; 69: 154–160.
Acknowledgements
We thank Ms Yukiko Lema for organizing the figures and manuscript. This work was supported by USPHS grants MH-084018 (AS), MH-094268 Silvo O Conte center (AS), MH-069853 (AS), MH-085226 (AS), MH-088753 (AS), MH-092443 (AS), MH-091387 (AS), MH-057683 (PO), MH-083728 (MP), Stanley (AS), RUSK (AS), S-R foundations (AS), NARSAD (AS, PO and MP), the Maryland Stem Cell Research Fund (AS), MEXT KAKENHI (TT), JST CREST (TT) and the Naito and Uehara Memorial Foundations (JN).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Nomura, J., Jaaro-Peled, H., Lewis, E. et al. Role for neonatal D-serine signaling: prevention of physiological and behavioral deficits in adult Pick1 knockout mice. Mol Psychiatry 21, 386–393 (2016). https://doi.org/10.1038/mp.2015.61
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.61
This article is cited by
-
Early interventions in risk groups for schizophrenia: what are we waiting for?
npj Schizophrenia (2016)