Abstract
Mammary analog secretory carcinoma (MASC) is a recently recognized low-grade salivary carcinoma characterized by a specific ETV6 rearrangement. We describe 14 new MASCs and examine their immunophenotypic and genetic profiles in the context of look-alikes, namely, low-and high-grade salivary duct carcinoma and acinic cell carcinoma. ETV6 rearrangement, and robust expression of mammaglobin and S100, were demonstrated in 11/11, 14/14, and 12/14 MASCs, respectively. All low-grade salivary duct carcinomas coexpressed S100/mammaglobin (6/6); none harbored ETV6 rearrangements (0/5). Given that S100/mammaglobin coexpression and absence of zymogen granules are features of both MASC and low-grade salivary duct carcinoma, these two are best distinguished histologically. The former is predominantly an extraductal neoplasm with bubbly pink cytoplasm, whereas the latter is a distinct intraductal micropapillary and cribriform process. Querying ETV6 gene status may be necessary for difficult cases. No acinic cell carcinoma expressed mammaglobin (0/13) or harbored an ETV6 rearrangement (0/7); only 1/13 acinic cell carcinomas weakly expressed S100. DOG1 expression was limited or absent among all tumor types, except acinic cell carcinoma which expressed DOG1 diffusely in a canalicular pattern. Therefore, histology and immunohistochemistry (mammaglobin, S100, DOG1) suffices in distinguishing acinic cell carcinoma from both MASC and low-grade salivary duct carcinoma. HER2 (ERBB2) amplification was detected in only 1/10 acinic cell carcinomas, but none of the MASCs or low-grade salivary duct carcinomas tested. High-grade salivary duct carcinomas frequently expressed mammaglobin (11/18) and harbored HER2 amplifications (13/15); none harbored ETV6 rearrangements (0/12). High-grade salivary duct carcinomas can easily be distinguished from these other entities by histology and HER2 amplification.
Similar content being viewed by others
Main
Mammary analog secretory carcinoma (MASC) is a recently recognized low-grade salivary carcinoma that shares the same histology and ETV6 gene (12p13) rearrangement as secretory carcinoma of the breast. Before its recognition by Skálová et al1 in 2010, MASC was frequently misclassified as acinic cell carcinoma or adenocarcinoma not otherwise specified. Historical diagnoses of extraparotid, or zymogen-poor, or papillocystic variants of acinic cell carcinomas are especially suspect for being misclassified.2, 3, 4 Mammaglobin/S100 coexpression have been touted as useful in diagnosing MASC, as nearly all examples express both biomarkers, whereas the vast majority of acinic cell carcinomas are negative.5, 6 However, the specificity of mammaglobin/S100 coexpression has not been extensively examined in low-grade salivary duct carcinoma, a MASC look-alike, or high-grade salivary duct carcinoma, a low-grade salivary duct carcinoma look-alike. Recently, DOG1 has been demonstrated as a good diagnostic biomarker for acinic cell carcinoma;7 it has not been extensively tested in low-grade salivary duct carcinoma or MASC. GATA3, a zinc-finger transcription factor, has recently been reported to be expressed by many salivary tumors including MASC,8 but has not been tested extensively in low-grade salivary duct carcinoma. We report the immunohistochemical profiling of MASC and its mimics, namely acinic cell carcinoma, low-grade salivary duct carcinoma, high-grade salivary duct carcinoma, and cystic low-grade mucoepidermoid carcinoma, with respect to mammaglobin, S100 protein, DOG1, and GATA3. We also interrogate this tumor set for the ETV6 gene rearrangement using break-apart fluorescence in situ hybridization for the 12p13 locus, and HER2 (ERBB2) gene amplification by chromogenic in situ hybridization.
Materials and methods
Salivary gland tumors diagnosed in the Department of Pathology, University of Alabama at Birmingham, between 2005 and 2012, and the consultation files of MBG, were reviewed. To identify any MASC cases from the pathology archives, we reviewed all diagnoses of acinic cell carcinoma, low-grade salivary duct carcinoma, low-grade carcinoma not otherwise specified, cystadenocarcinoma, and cystadenoma. The following criteria were used for the diagnosis of MASC: low-grade salivary carcinoma with bubbly eosinophilic cytoplasm, no zymogen granules, plus mammaglobin/S100 coexpression. Features of low-grade salivary duct carcinoma (eg, intraductal cribriform and/or micropapillary proliferations) were absent. All low-grade salivary duct carcinomas were included for study. Select acinic cell carcinomas, cystic low-grade mucoepidermoid carcinomas, and high-grade salivary duct carcinomas were also included. Diagnoses of these entities were established based on published criteria.3, 4, 9, 10, 11
Hematoxylin and eosin-stained slides were examined, and 4 μm tumor sections were cut from archival tissue blocks. Immunohistochemistry was performed for mammaglobin, S100 protein, DOG1, and GATA3 (Table 1) after heat-induced epitope retrieval; secondary antibody- and horseradish peroxidase-labeled polymer technology with 3,3’-diaminobenzidene (DAB) served as the chromogenic substrate.
Fluorescence in situ hybridization using a break-apart probe for the ETV6 (12p13) gene was performed on 4–6 μm unstained sections prepared from archival tissue blocks. The LSI® ETV6 (12p13) Dual-Color Break-Apart Rearrangement Probe (Abbott Molecular, Des Plaines, IL, USA) was used to interrogate the loci of interest. Slides were pretreated using an automated VP 2000™ processor (Abbott Molecular) with manufacturer’s protocols. Cellular DNA and probes were co-denatured at 76 °C for 10 min using the ThermoBrite™ system (Abbott Molecular) and incubated overnight at 37 °C in a humidified chamber. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI) in Antifade solution (Abbott Molecular), and slides were analyzed using a Leica DM6000B fluorescence microscope (Leica Microsystems, Buffalo Grove, IL, USA). Hybridization signals were assessed in 50–100 interphase tumor nuclei per specimen by two authors (JS and PA). Cutoff value for rearrangement (split red and green signals) was ≥15%, for gain (≥3 normally juxtaposed ETV6 signals) was ≥10%, and for loss (1 copy of a normally juxtaposed ETV6 signal) was ≥25%. Amplification of 12p13 could not be detected as no centromeric chromosome 12 probe was used. Images were acquired and archived using the CytoVision Image Analysis System (Genetix, San Jose, CA, USA).
Chromogenic in situ hybridization for the HER2 gene was performed using the Ventana INFORM HER2 Dual ISH DNA Probe Cocktail (Ventana Medical Systems, Tucson, AZ, USA) and a Ventana auto-stainer. HER2/chromosome 17 ratios were determined by examining 20 interphase tumor nuclei and counting HER2 gene (red) and centromeric chromosome 17 (black) signals and then determining the average ratio. If HER2/chromosome 17 ratio was >2.2, then HER2 was considered amplified; if HER2/chromosome 17 was <1.8, then HER2 was considered unamplified. If HER2/chromosome17 was ≥1.8 but ≤2.2, then 20 additional tumor nuclei were examined; a new HER2/chromosome 17 ratio was calculated based on the sum of 40 nuclei. If the new ratio was ≥2.0, then HER2 was considered amplified; if the new ratio was <2.0, then HER2 was considered unamplified.
Results
Mammary Analog Secretory Carcinoma
Demographics
Fourteen MASCs were identified: 6 males and 8 females. The median patient age was 54.5 years (range: 22–80; mean: 55.3). Nine MASCs were from parotid, two arose from the lip, and one each originated in the submandibular gland, hard palate, and thyroid; this latter case is assumed to have arisen from regional ectopic salivary tissue.
Histology
Architecturally, MASC revealed varying proportions of solid, microcystic, tubular, papillocystic, and cribriform growth patterns (Figure 1). A variable degree of infiltration into surrounding tissues was present in 10 cases. Four MASCs were noninfiltrative, one of which was characterized by multiple macrocysts with intracystic proliferation (Figure 2a and b), and three of which were entirely unicystic (Figure 2c–e). The macrocystic/microcystic pattern is associated with hemorrhage, hemosiderin, and cholesterol clefts, akin to low-grade salivary duct carcinoma. When true intraductal MASC was present, it was focal and revealed a solid growth pattern (Figure 3a and b). This feature is useful when distinguishing MASC from low-grade salivary duct carcinoma.
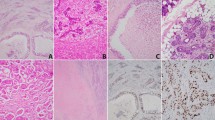
Cystic mammary analog secretory carcinoma (MASC). (a, b) Multicystic MASC may mimic low-grade salivary duct carcinoma. (c, d, e) This unicystic MASC was originally classified as a zymogen-poor cystic acinic cell carcinoma. (d) If one is considering the diagnosis of zymogen-poor acinic cell carcinoma, then look among the tumor cells for scattered basophilic zymogen cells. None are seen here. (e) Strong mammaglobin expression. (f) Another MASC with bubbly vacuolated cytoplasm. Interspersed clear cells are seen, but no basophilic zymogen-containing cells are present.
Mammary analog secretory carcinoma (MASC). Focal intraductal tumor can be seen. (a) Intraductal MASC as a mural plaque. (b) Solid proliferation of MASC beneath intact ductal epithelium. Cytologically, the neoplastic cells displayed small, low-grade nuclei with abundant pink frothy cytoplasm. (c, d) Microcystic MASC with mucin production. (e) Intracellular mucicarmine-positive secretions. This tumor was mammaglobin positive and S100 negative, raising the consideration of mucoepidermoid carcinoma. ETV6 gene rearrangement was present, confirming the diagnosis of MASC.
All MASCs were cytologically low grade with uniform cells, small- to medium-sized nuclei, occasional small nucleoli, and abundant pink, bubbly cytoplasm. No cytoplasmic zymogen granules were seen. Tumor cells secrete mucin-like and/or eosinophilic material (Figure 3c and d). One lip MASC (case 4) contained goblet cells with intracellular mucicarmine-positive secretions (Figure 3e). This tumor was mammaglobin positive/S100 negative, raising the consideration of mucoepidermoid carcinoma. However, no squamoid or intermediate-type cells were present; ETV6 gene rearrangement was confirmed in this case.
Mitotic figures were rarely encountered; when present they were ≤3 mitotic figures per 10 high-power fields. No lymphovascular invasion was observed. Perineural invasion was seen in one MASC. Tumor necrosis was present in four MASCs; one case also revealed more high-grade cytology. This patient (case 1) developed a lung metastasis 4 years after initial diagnosis (Figure 4) of a parotid primary.
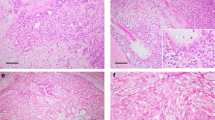
Parotid mammary analog secretory carcinoma (MASC; case 1, Table 2) with necrosis and increased cytologic grade (a, b, c). (d) This patient developed pulmonary metastases after 4 years. (e) Low-power view of this tumor demonstrates significant DOG1 expression that may raise the consideration of acinic cell carcinoma. However, ETV6 rearrangement was confirmed in both the primary and metastatic MASCs. (f) High-power view demonstrates that DOG1 expression is concentrated at the periphery of tumor nests, or decorates glandular lumina. In contrast, acinic cell carcinoma demonstrates diffuse canalicular DOG1 expression pattern (see Figure 7).
One MASC was highly unusual in that it infiltrated the thyroid and perilaryngeal soft tissues; the site of origin may have been ectopic minor salivary glands (case 2) (Figure 5a–c). This MASC was confirmed by ETV6 translocation (Figure 5d), coexpressed mammaglobin and S100, and was negative for TTF1 and thyroglobulin.
(a–d) Mammary analog secretory carcinoma (MASC) invading thyroid gland (case 2, Table 2). (b) Mammaglobin expression. (d) Fluorescence in situ hybridization for ETV6 rearrangement reveals distinct separated red and green signals (arrows) indicating a 12p13 gene rearrangement. The yellow signals represent the intact gene copy. (e) Example of low-grade salivary duct carcinoma that is translocation negative. Here the arrows indicate two signals per cell, either yellow, or closely approximated green and red, indicative of the intact gene. (f) Example of high-grade salivary duct carcinoma with three or more approximated green and red signals per cell indicative of ETV6 gain.
Immunohistochemistry and HER2 (ERBB2)
All MASCs diffusely expressed mammaglobin, except for one case with focal expression (case 3). This case was positive for an ETV6 rearrangement (Figure 6). S100 expression was present in 12/14 MASCs. DOG1 expression was usually limited to cells at the tumor island periphery, usually in a membranous and apical pattern. Ten MASCs were tested and found to have a nonamplified HER2 gene by chromogenic in situ hybridization.
Mammary analog secretory carcinoma showing limited mammaglobin expression (MASC; case 3, Table 2). (a, b) Resection of residual parotid MASC that extends into subcutaneous tissues. (c, d) Only focal yet strong mammaglobin expression was seen in the original excision. Immunohistochemistry for mammaglobin from the resection specimen (not shown) was negative. (e) DOG1 expression was limited to tumor island peripheries, with a membranous distribution (inset). (f) Strong, diffuse S100 expression. This profile excludes acinic cell carcinoma. (g) This limited mammaglobin expressing MASC case showed an ETV6 rearrangement in 70% of tumor cells.
ETV6
Eleven MASCs were tested and the ETV6 gene rearrangement was confirmed in all cases; 70–99% (mean 88.5%) of tumor nuclei demonstrated the ETV6 gene break. One case revealed a mosaic pattern of ETV6 rearrangement (48%) and ETV6 rearrangement and additional copy of 12p13 (46%). Three MASCs were not tested by fluorescence in situ hybridization, as the histopathology and mammaglobin/S100 coexpression were sufficiently diagnostic. The salient features of our MASCs are presented in Table 2.
Acinic Cell Carcinoma
Demographics
Thirteen acinic cell carcinomas (Figure 7) were studied (4 men and 9 women; median age: 63; mean age: 61.7; range: 39–76 years, all from parotid gland).
(a) Microcystic acinic cell carcinoma. (b) The microcystic pattern and bubbly cytoplasm is reminiscent of mammary analog secretory carcinoma (MASC), although ‘too blue’. (c) Acinic cell carcinoma demonstrating intercalated duct differentiation. (d) Even in this ‘pink acinic cell carcinoma,’ acinar differentiation (zymogen granules) can be found. (e–g) This unusual acinic cell carcinoma was extensively infiltrative, solid, microcystic, with comedonecrosis (e) and revealed intermediate- to high-grade nuclei and pale to clear vacuolated cytoplasm (f) and showed strong, diffuse DOG1 expression with a canalicular pattern (g). The differential diagnosis included MASC, high-grade salivary duct carcinoma, and zymogen-poor acinic cell carcinoma. There was no expression of S100 or mammaglobin, and HER2 was not amplified (not shown). The final diagnosis was acinic cell carcinoma. (h) Low-power view of diffuse, strong DOG1 expression in a classic acinic cell carcinoma. (i) Higher power of same tumor demonstrating cytoplasmic and membranous DOG1 expression.
Histology
All acinic cell carcinomas demonstrated varying mixtures of solid, microcystic, tubular, and papillocystic architecture, and variable invasion. By definition, acinic cell carcinomas contained, at the very least, scattered cells with cytoplasmic zymogen granules (Figure 7d).
Immunohistochemistry, HER2 (ERBB2), and ETV6
Expression pattern of DOG1 was characteristically strong, diffuse, cytoplasmic, often in a canalicular pattern (Figure 7g). All acinic cell carcinomas were mammaglobin negative, and only one was weakly positive for S100. One of 10 acinic cell carcinomas tested revealed HER2 amplification. Seven acinic cell carcinomas were tested and found negative for ETV6 gene rearrangement. One acinic cell carcinoma did show loss of one copy of 12p13 in 38% of tumor cells.
One interesting acinic cell carcinoma was extensively infiltrative, solid, microcystic, with necrosis (Figure 7e–g). Tumor cells had intermediate- to high-grade nuclei and pale to clear cytoplasm with focal suggestions of zymogen granules. The differential diagnosis included MASC, high-grade salivary duct carcinoma, and zymogen-poor acinic cell carcinoma. Immunohistochemistry for DOG1 revealed strong, diffuse staining with a canalicular pattern; no expression of S100 or mammaglobin, and nonamplified HER2 gene. The final diagnosis was acinic cell carcinoma.
Low-Grade Salivary Duct Carcinoma
Demographics
Six low-grade salivary duct carcinomas were identified, with patient age available in five cases (median age: 61; mean age: 58.6; range: 39–78 years). All arose from the parotid. Gender was available in four cases: two males and two females.
Histology
Briefly, low-grade salivary duct carcinomas revealed a predominant intraductal growth pattern composed of low-grade ductal epithelial cells forming filigreed and anastomosing micropapillae with a fenestrated appearance (Figure 8a–d). Calponin expression confirmed the intraductal component; extraductal invasion was documented in one case. Cytologically, tumor cells contained bland nuclei with evenly distributed chromatin and inconspicuous nuclei. Cell cytoplasm was eosinophilic to apocrine-like, with occasional yellow, lipofuscin-like cytoplasmic pigment. Interestingly, one in situ low-grade micropapillary salivary duct carcinoma was associated with a microscopic focus of epithelial cells in a periparotid lymph node. This particular tumor demonstrated some areas with intermediate-grade nuclei, but no progression to high grade or invasion was found (Figure 9). It is likely that the lymph node finding represents an epithelial inclusion from micropapillae rather than occult metastases. This patient declined radiation therapy and shows no evidence of recurrence 13 months after surgery.
Low-grade salivary duct carcinoma. (a) Characteristically, low-grade salivary duct carcinoma reveals distinctly separated cystically dilated ducts, intraductal proliferations, hemorrhage, and hemosiderin. (b) Micropapillary intraductal proliferations. (c) Pseudopapillae with bland hobnail cells. (d) Bland cuboidal cells with eosinophilic cytoplasm. Microvacuoles can be seen, however, the generalized bubbliness of mammary analog secretory carcinoma (MASC) is absent. (e) Diffuse strong mammaglobin expression. (f) DOG-1 expression is limited to duct periphery and basal/parabasal cells.
This low-grade salivary duct carcinoma is highly unusual because of a finding in a periparotid lymph node. (a) Typical low-power appearance of cystically dilated ducts with intracystic proliferation. (b) Micropapillary proliferation. (c) Calponin staining revealed intact ducts, with no evidence of invasion. (d) Intermediate-grade nuclei. (e) Epithelial lymph node inclusion (see discussion). (f) Cytokeratin expression by lymph node inclusion.
Immunohistochemistry, HER2 (ERBB2), and ETV6
All low-grade salivary duct carcinomas demonstrated diffuse, strong expression of S100 protein as well as mammaglobin (Figure 8e). DOG1 expression was limited to cells at the periphery of the ducts in all cases (Figure 8f). Five low-grade salivary duct carcinomas were tested and found negative for ETV6 rearrangement (Figure 5e), including the case with a small epithelial inclusion in a lymph node. All low-grade salivary duct carcinomas were nonamplified with respect to the HER2 gene.
High-Grade Salivary Duct Carcinoma
Demographics
Eighteen high-grade salivary duct carcinomas were studied; patient age and tumor site were known for 13 cases (median age: 63; mean age: 65.8; range: 44–96 years, 12 parotid, 1 buccal). Gender was available in 12 cases; 9 males and 3 females.
Histology, immunohistochemistry, HER2 (ERBB2), and ETV6
High-grade salivary duct carcinomas were typically widely invasive, with the exception of one case (Figure 10). The invasive growth pattern was either solid or glandular cribriform. The ductal carcinoma in situ component, when present, characteristically mimicked high-grade breast ductal carcinoma in situ with comedonecrosis and cribriform pattern. Carcinoma cells were uniformly high grade with prominent nucleoli, coarse chromatin, and abundant amphophilic to apocrine cytoplasm, often with well-defined cell borders.
Two high-grade salivary duct carcinoma cases arose from pleomorphic adenomas. One buccal high-grade salivary duct carcinoma (Figure 10) was previously diagnosed as high-grade cystadenocarcinoma, not otherwise specified, but was reclassified as in situ high-grade salivary ductal carcinoma based on an amplified HER2 gene.
HER2 amplification was present in 13/15 cases. Mammaglobin expression was seen in 11/18 cases, with diffuse, strong expression in 7 tumors. S100 protein was expressed in 2/18 cases. DOG1 was expressed in 5/17 tumors usually with a membranous pattern. Twelve tumors were tested and found negative for ETV6 rearrangement. Interestingly, however, 11 cases showed gains of the 12p13 gene (Figure 5f) identified in 14–87% of tumor cells; mean percentage of tumor nuclei with 12p13 gain: 43.7%.
Low-Grade Mucoepidermoid Carcinoma
Two low-grade mucoepidermoid carcinomas demonstrated mammaglobin expression limited to goblet cells. However, these cases were negative for S100, and were otherwise typical for mucoepidermoid carcinoma. One parotid cystadenoma showed diffuse S100 expression, focal mammaglobin expression, but was negative for the ETV6 gene break by fluorescence in situ hybridization. The immunohistochemical expression patterns and ETV6 (12p13) and HER2 gene status are summarized in Table 3.
Discussion
In 2010, MASC was described as a low-grade salivary tumor composed of carcinoma cells with pink vacuolated cytoplasm forming microcystic, tubular, or solid patterns, and uniformly expressing mammaglobin and S100 protein. The seminal report by Skálová et al1 included testing for the ETV6 gene rearrangement by fluorescence in situ hybridization and the ETV6/NTRK3 fusion by reverse transcriptase–PCR. All 11 MASCs revealed ETV6 gene rearrangement; ETV6-NTRK3 fusion transcripts were confirmed in 13/14 tumors.1 The recurrent t(12;15)(p13;q25) rearrangement results in the fusion of ETV6, a transcriptional regulator on 12p13, with NTRK3, which encodes a membrane tyrosine kinase receptor.12, 13 This same ETV6/NTRK3 fusion is also characteristic of secretory carcinoma of the breast, a tumor that is histologically identical to MASC, as well as congenital mesoblastic nephroma, congenital fibrosarcoma, and some forms of acute myeloid leukemia.6
Subsequent reports of MASC broadened the histologic range of this neoplasm, as macrocystic, polypoid, and papillocystic patterns and perineural invasion were described.13 Intraductal growth pattern, expression of high-molecular-weight keratin, and focal mucin production were described, suggesting that low-grade mucopidermoid carcinoma should be considered in the differential diagnosis.13 Intraoral MASCs were described.2, 13, 14, 15 Half of intraoral MASCs were found to be S100 negative, suggesting that oral acinic cell carcinomas may not even exist.13 This argument was put to rest in a study examining 14 extraparotid ‘acinic cell carcinomas’ that were interrogated for the ETV6 gene rearrangement by break-apart fluorescence in situ hybridization. Of these 14 extraparotid ‘acinic cell carcinomas,’ 11 demonstrated ETV6 gene breaks and were reclassified as MASCs.2 Three ETV6 translocation-negative intraoral tumors demonstrated basophilic cytoplasmic granules, thus validating the original diagnosis of extraparotid acinic cell carcinoma.2 Therefore, intraoral acinic cell carcinomas still do exist in the post-MASC era of salivary gland pathology.
In 2014, Skálová et al16 reviewed 100 MASCs and found 3 with high-grade transformation; these cases demonstrated typical MASC adjacent to high-grade carcinoma with trabecular architecture, cytologic anaplasia, prominent nucleoli, decreased bubbly secretions, and comedonecrosis. Both typical MASC areas and the high-grade components demonstrated ETV6 gene rearrangements and similar mammaglobin/S100 expression profiles. Two of these three high-grade MASC cases demonstrated lymph node metastasis and all three died of disease.
Before its recognition, MASC was often misclassified as zymogen-poor acinic cell carcinoma. Some of the most prominent published series of acinic cell carcinoma likely harbored misclassified cases; reevaluation of the prognosis of acinic cell carcinoma in the post-MASC is therefore warranted. The prognosis of MASC is not yet completely known. Two studies have explored this issue.3, 4 Seethala and colleagues3 reviewed 81 cases previously diagnosed as acinic cell carcinoma, reclassifying 10 zymogen-poor tumors as MASC, confirmed by ETV6 gene rearrangement. Seven zymogen-poor tumors lacked the ETV6 rearrangement and were left as acinic cell carcinomas. High-grade transformation was demonstrated in 11 acinic cell carcinomas, 4 of which were also tested and were negative for the ETV6 rearrangement. With respect to outcome, mean survivals for classic acinic cell carcinoma and acinic cell carcinoma with high-grade transformation were 125 and 40.2 months, respectively. Lymph node metastases were more common in acinic cell carcinoma with high-grade transformation (2/5, 40%), and MASC (2/6, 33.3%) as compared with classic acinic cell carcinoma (3/38, 7.9%). In a separate paper,4 the rates of lymph node metastasis for MASC and acinic cell carcinoma were 17.6% (6/34) and 7.9% (3/38), respectively. This paper also found no significant differences in mean disease-free survivals for MASC and acinic cell carcinoma (92 and 121 months, respectively). The authors concluded that MASC and acinic cell carcinoma can probably be treated similarly.
The 14 new MASCs herein bring the total number of reported MASCs to at least 197 cases, affecting patients aged 13–86 years, with a male/female ratio of 1.2:1.1, 2, 3, 4, 5, 6, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 Among the reported cases with definite tumor sites, 123 involved the parotid, 28 arose from oral cavity, 13 arose from the submandibular gland, and 9 from the lip. Here we report the first case of a MASC involving the thyroid gland. We also report the first case, to our knowledge, of MASC metastatic to the lung.
Mammaglobin expression has been touted as characteristic of MASC, but few studies have explored the specificity of this biomarker for salivary tumors. Chernock and colleagues39 found strong mammaglobin expression in 15/15 polymorphous low-grade adenocarcinomas, 7/15 adenoid cystic carcinomas, 8/15 mucoepidermoid carcinomas, 2/2 adenocarcinoma, not otherwise specified, and 1 mucinous adenocarcinoma. Mammaglobin/S100 coexpression was demonstrated in nine polymorphous low-grade adenocarcinomas and two adenoid cystic carcinomas, all of which were confirmed as negative for the ETV6 gene rearrangement.39
Westra and colleagues5 tested 131 salivary gland tumors for the ETV6 gene break by fluorescence in situ hybridization and identified 15 MASCs, all of which were translocation positive, with strong, diffuse mammaglobin expression. However, many ETV6 rearrangement-negative salivary gland tumors also expressed mammaglobin, including one low-grade salivary duct carcinoma and some polymorphous low-grade adenocarcinomas, high-grade salivary duct carcinomas, mucoepidermoid carcinomas, and pleomorphic adenomas. These authors also commented on some histologic overlap between low-grade salivary duct carcinoma and MASC. Parenthetically, before this paper, only 10 low-grade salivary duct carcinomas, to our knowledge, have been tested for the ETV6 rearrangement; all have been negative.1, 3, 4, 5, 6, 37
Low-grade salivary duct carcinoma9, 10 is a rare neoplasm resembling low-grade cribriform ductal carcinoma in situ of the breast. It is characterized by intraductal proliferation of low-grade ductal cells with filigreed, anastomosing micropapillae, loose cribriform, occasional solid patterns, and lipofuscin-like cytoplasmic pigment. Limited extraductal invasion can be seen. Typically low-grade salivary duct carcinoma has a nonaggressive clinical course.9 No recurrences have been reported to date.40 Metastatic disease has been reported in only one unusual low-grade salivary duct carcinoma that demonstrated progression to high-grade intraductal and invasive salivary duct carcinoma, with multiple regional metastases of high-grade carcinoma.41 As benign epithelial inclusions and epithelial displacements in lymph nodes from intraductal papillary or micropapillary lesions are known to occur, we conclude that our case of in situ low-grade salivary duct carcinoma with an associated epithelial island in a periparotid lymph node represents a benign epithelial lymph node inclusion or epithelium displacement rather than an occult metastasis. High-grade salivary duct carcinoma can mimic low-grade salivary duct carcinoma, but is a highly malignant, widely invasive adenocarcinoma with oncocytoid cytoplasm, high-grade nuclei, and overexpression of HER2,9, 10, 42 unlike low-grade salivary duct carcinoma.
Consistent with other reports, we find that mammaglobin expression is sensitive for MASC, but not specific, as it is also expressed in all low-grade salivary duct carcinomas, many high-grade salivary duct carcinomas, and by the goblet cells of low-grade mucoepidermoid carcinoma. Importantly, mammaglobin expression may be limited in MASC, as one of our molecularly confirmed MASC cases showed very focal mammaglobin expression (case 3, Figure 6); the second resection specimen for tumor persistence was completely mammaglobin negative. All acinic cell carcinomas tested were mammaglobin negative. We confirm that immunohistochemistry for mammaglobin and S100 can distinguish most MASCs and low-grade salivary duct carcinomas from acinic cell carcinoma; only two MASCs were S100 negative and one acinic cell carcinoma was S100 protein positive. Limited DOG1 expression was seen in many of the tumors studied. However, the pattern of DOG1 expression was useful for confirming the diagnosis of acinic cell carcinoma. Acinic cell carcinomas demonstrate strong, diffuse cytoplasmic and canalicular expression of DOG1. Both MASC and low-grade salivary duct carcinoma show weaker DOG1 expression limited to the cells at the periphery of tumor groups. GATA3 expression was not found to be useful in this differential diagnostic context.
We also confirm that the ETV6 gene rearrangement can distinguish MASC from low- and high-grade salivary duct carcinomas and acinic cell carcinoma. All MASCs tested demonstrated the ETV6 gene rearrangement, whereas all other salivary tumors tested were negative for the rearrangement. Interestingly, most high-grade salivary duct carcinomas showed 12p13 gains, warranting further study. HER2 amplification is common in high-grade salivary duct carcinomas. No MASC or low-grade salivary duct carcinoma demonstrated HER2 gene amplification.
Histopathology is the best discriminator between MASC and low-grade salivary duct carcinoma. MASC is predominantly infiltrating, with a variable cystic component and composed of tumor cells with abundant pink, bubbly cytoplasm, eosinophilic to mucinous secretions, and solid, microcystic, tubular, papillocystic, and trabecular architectures. On the other hand, low-grade salivary duct carcinoma is typically an intraductal multicystic proliferation. Calponin staining specifically confirms the intraductal component. The intraductal/intracystic tumor is composed of low-grade cells with micropapillary and sieve-like fenestrated architecture. Tumor cells may contain focal yellow cytoplasmic pigment. No bubbly cytoplasm, basophilic zymogen granules, or mucinous secretions are seen. Another helpful point is that low-grade salivary duct carcinoma, unlike MASC, rarely involves minor salivary glands. Despite histologic and immunophenotypic overlap, it can be confidently stated that MASC and low-grade salivary duct carcinoma are indeed molecularly distinct. A total of 15 low-grade salivary duct carcinomas have now been negative for the ETV6 gene rearrangement. If the distinction between MASC and low-grade salivary duct carcinoma remains problematic, then fluorescence in situ hybridization for the ETV6 rearrangement can be the gold standard. Strong and diffuse mammaglobin/S100 protein coexpression definitely excludes acinic cell carcinoma. Strong cytoplasmic and canalicular expression of DOG1 definitely excludes MASC and low-grade salivary duct carcinoma. HER2 gene amplification may help separate high-grade salivary duct carcinoma from its mimics.
References
Skálová A, Vanecek T, Sima R et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol 2010;34;599–608.
Bishop JA, Yonescu R, Batista D et al. Most non-parotid “acinic cell carcinomas” represent mammary analog secretory carcinomas. Am J Surg Pathol 2013;37;1053–1057.
Chiosea SI, Griffith C, Assaad A et al. The profile of acinic cell carcinoma after recognition of mammary analog secretory carcinoma. Am J Surg Pathol 2012;36;343–350.
Chiosea SI, Griffith C, Assaad A et al. Clinicopathological characterization of mammary analogue secretory carcinoma of salivary glands. Histopathology 2012;61;387–394.
Bishop JA, Yonescu R, Batista D et al. Utility of mammaglobin immunohistochemistry as a proxy marker for the ETV6-NTRK3 translocation in the diagnosis of salivary mammary analogue secretory carcinoma. Hum Pathol 2013;44;1982–1988.
Shah AA, Wenig BM, LeGallo RD et al. Morphology in conjunction with immunohistochemistry is sufficient for the diagnosis of mammary analogue secretory carcinoma. Head Neck Pathol 2015;9;85–95.
Chenevert J, Duvvuri U, Chiosea S et al. DOG1: a novel marker of salivary acinar and intercalated duct differentiation. Mod Pathol 2012;25;919–929.
Schwartz LE, Begum S, Westra WH et al. GATA3 immunohistochemical expression in salivary gland neoplasms. Head Neck Pathol 2013;7;311–315.
Brandwein-Gensler M, Hille J, Wang BY et al. Low-grade salivary duct carcinoma. description of 16 cases. Am J Surg Pathol 2004;28;1040–1044.
Delgado R, Klimstra D, Albores-Saavedra J . Low-grade salivary duct carcinoma. A distinctive variant with a low-grade histology and a predominant intraductal growth pattern. Cancer 1996;78;958–967.
Brandwein MS, Ivanov K, Wallace DI et al. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histologic grading. Am J Surg Pathol 2001;25;835–845.
Skálová A . Mammary analogue secretory carcinoma of salivary gland origin: an update and expanded morphologic and immunohistochemical spectrum of recently described entity. Head Neck Pathol 2013;7;S30–S36.
Connor A, Perez-Ordonez B, Shago M et al. Mammary analog secretory carcinoma of salivary gland origin with the ETV6 gene rearrangement by FISH: expanded morphologic and immunohistochemical spectrum of a recently described entity. Am J Surg Pathol 2012;36;27–34.
Fehr A, Loning T, Stenman G . Mammary analogue secretory carcinoma of the salivary glands with ETV6-NTRK3 gene fusion. Am J Surg Pathol 2011;35;1600–1602.
Kratochvil FJ, Stewart JCB, Moore SR . Mammary analog secretory carcinoma of salivary glands: a report of 2 cases in the lips. Oral Surg Oral Med Oral Pathol Oral Radiol 2012;114;630–635.
Skálová A, Vanecek T, Majewska H et al. Mammary analogue secretory carcinoma of salivary glands with high-grade transformation. Report of 3 cases with the ETV6-NTRK3 gene fusion and analysis of TP53, beta-catenin, EGFR, and CCND1 genes. Am J Surg Pathol 2014;38;23–33.
Rastatter JC, Jatana KR, Jennings LJ et al. Mammary analogue secretory carcinoma of the parotid gland in a pediatric patient. Otolaryngol Head Neck Surg 2012;146;515–515.
Levine P, Fried K, Krevitt LD et al. Aspiration biopsy of mammary analogue secretory carcinoma of accessory parotid gland: another diagnostic dilemma in matrix-containing tumors of the salivary glands. Diagn Cytopathol 2012;42;49–53.
Petersson F, Lian D, Chau YP et al. Mammary analogue secretory carcinoma: the first submandibular case reported including findings on fine needle aspiration cytology. Head Neck Pathol 2012;6;135–139.
Lei Y, Chiosea SI . Re-evaluating historic cohort of salivary acinic cell carcinoma with new diagnostic tools. Head Neck Pathol 2012;6;166–170.
Griffith C, Seethala R, Chiosea SI . Mammary analogue secretory carcinoma: a new twist to the diagnostic dilemma of zymogen granule poor acinic cell carcinoma. Virchows Arch 2011;459;117–118.
Ito S, Ishida E, Skálová A et al. Case report of mammary analog secretory carcinoma of the parotid gland. Pathol Int 2012;62;149–152.
Pisharodi L . Mammary analog secretory carcinoma of salivary gland: cytologic diagnosis and differential diagnosis of an unreported entity. Diagn Cytopathol 2012;41;239–241.
Jung Jung M, Seon Song J, Kim SY et al. Finding and characterizing mammary analogue secretory carcinoma of the salivary gland. Korean J Pathol 2013;47;36–43.
Williams L, Chiosea SI . Mammary analogue secretory carcinoma mimicking salivary adenoma. Head Neck Pathol 2013;7;316–319.
Bishop JA, Yonescu R, Batista DA et al. Cytopathologic features of mammary analogue secretory carcinoma. Cancer Cytopathol 2013;121;228–233.
Griffith CC, Stelow EB, Saqi A et al. The cytological features of mammary analogue secretory carcinoma. A series of 6 molecularly confirmed cases. Cancer Cytopathol 2013;121;234–241.
Laco J, Svajdler M, Andrejs J et al. Mammary analog secretory carcinoma of salivary glands: a report of 2 cases with expression of basal/myoepithelial markers (calponin, CD10 and p63 protein). Pathol Res Pract 2013;209;167–172.
Cooper D, Burkey B, Chute D et al. Mammary analogue secretory carcinoma of the soft palate: a report of two cases. Int J Otolaryngol Head Neck Surg 2013;2;174–178.
Mariano FV, dos Santos HT, Azanero WD et al. Mammary analogue secretory carcinoma of salivary glands is a lipid rich tumor, and adipophilin can be valuable in its identification. Histopathology 2013;63;558–567.
Sethi R, Kozin E, Remenschneider A et al. Mammary analogue secretory carcinoma: update on a new diagnosis of salivary gland malignancy. Laryngoscope 2014;124;188–195.
Woo J, Seethala RR, Sirintrapun SJ . Mammary analogue secretory carcinoma of the parotid gland as a secondary malignancy in a childhood survivor of atypical teratoid rhabdoid tumor. Head Neck Pathol 2014;8;194–197.
Hwang MJ, Ru WuP, Chen CM et al. A rare malignancy of the parotid gland in a 13-year-old Taiwanese boy: case report of a mammary analogue secretory carcinoma of the salivary gland with molecular study. Med Mol Morphol 2014;47;57–61.
Projetti F, Lacroix-Triki M, Serrano E et al. A comparative immunohistochemistry study of diagnostic tools in salivary gland tumors: usefulness of mammaglobin, gross cystic disease fluid protein 15, and p63 cytoplasmic staining for the diagnosis of mammary analog secretory carcinoma? J Oral Pathol Med 2014;44;244–251.
Majewska H, Skálová A, Stodulski D et al. Mammary analogue secretory carcinoma of salivary glands: a new entity associated with ETV6 gene rearrangement. Virchows Arch 2015;466;245–254.
Pinto A, Nose V, Rojas C et al. Searching for mammary analog secretory carcinoma of salivary gland among its mimics. Mod Pathol 2014;27;30–37.
Urano M, Nagao T, Miyabe S et al. Characterization of mammary analogue secretory carcinoma of the salivary gland: discrimination from its mimics by the presence of the ETV6-NTRK3 translocation and novel surrogate markers. Hum Pathol 2015;46;94–103.
Serrano-Arevalo ML, Mosqueda-Taylor A, Dominguez-Malagon H et al. Mammary analogue secretory carcinoma (MASC) of salivary gland in four Mexican patients. Med Oral Patol Oral Cir Bucal 2015;20;e23–e29.
Patel KR, Solomon IH, El-Mofty SK et al. Mammaglobin and S-100 immunoreactivity in salivary gland carcinomas other than mammary analogue secretory carcinoma. Hum Pathol 2013;44;2501–2508.
Brandwein-Gensler M, Wei S . Envisioning the next WHO head and neck classification. Head Neck Pathol 2014;8;1–15.
Weinreb I, Tabanda-Lichauco R, Van der Kwast T et al. Low-grade intraductal carcinoma of salivary gland: report of 3 cases with marked apocrine differentiation. Am J Surg Pathol 2006;30;1014–1021.
Clauditz TS, Reiff M, Gravert L et al. Human epidermal growth factor receptor 2 (HER2) in salivary gland carcinomas. Pathology 2011;43;459–464.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Stevens, T., Kovalovsky, A., Velosa, C. et al. Mammary analog secretory carcinoma, low-grade salivary duct carcinoma, and mimickers: a comparative study. Mod Pathol 28, 1084–1100 (2015). https://doi.org/10.1038/modpathol.2015.64
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2015.64
This article is cited by
-
Secretory carcinoma of the salivary gland is rich in lactoferrin: a possible lactational-like differentiation?
European Archives of Oto-Rhino-Laryngology (2023)
-
Identification of LMO2 as a new marker for acinic cell carcinoma of salivary gland
Diagnostic Pathology (2022)
-
Secretory Carcinoma of the Oral Cavity: A Retrospective Case Series with Review of Literature
Head and Neck Pathology (2021)
-
Salivary-Like Tumors of the Thyroid: A Comprehensive Review of Three Rare Carcinomas
Head and Neck Pathology (2021)
-
A rare case of high-grade intraductal carcinoma of the upper lip: immunohistochemical and genetic analyses
Medical Molecular Morphology (2021)