Abstract
Merkel cell carcinoma of the skin is a malignant neuroendocrine tumour, whose prognostic criteria are a matter of dispute. Specifically, no predictor is presently available in stage I–II tumours. We collected clinical and follow-up data from 70 Merkel cell carcinomas of the skin. The same cases were studied for p63 expression by immunohistochemistry, by reverse-transcription PCR (RT-PCR) and TP63 gene status by FISH and for presence of Merkel cell polyomavirus by PCR. Stage emerged as a significant prognostic parameter (P=0.008). p63 expression, detected in 61% (43/70) of cases by immunohistochemistry, was associated with both decreased overall survival (P<0.0001) and disease-free survival (P<0.0001). Variable expression patterns of the different p63 isoforms were found only in cases immunoreactive for p63. In these latter lesions, at least one of the N-terminal p63 isoforms was detected and TAp63α was the most frequently expressed isoform. TP63 gene amplification was observed by FISH in only one case. Presence of Merkel cell polyomavirus DNA sequences was detected in 86% (60/70) of Merkel cell carcinomas and did not emerge as a significant prognostic parameter. Merkel cell carcinoma cases at low stage (stage I-II) represented over half (40/70 cases, 57%) of cases, and the clinical course was uneventful in 25 of 40 cases while 15 cases died of tumour (10/40 cases) within 34 months or were alive with disease (5/40 cases) within 20 months. Interestingly, a very strict correlation was found between evolution and p63 expression (P<0.0001). The present data indicate that p63 expression is associated with a worse prognosis in patients with Merkel cell carcinoma, and in localised tumours it represents the single independent predictor of clinical evolution.
Similar content being viewed by others
Main
Merkel cell carcinoma of the skin is an aggressive neuroendocrine tumour more lethal than melanoma, as no fewer than one-third of Merkel cell carcinoma patients die of this cancer.1 Although Merkel cell carcinoma, which primarily affects elderly individuals, is rare, its incidence is rapidly increasing.1 In contrast to the noticeable advances that have explained the molecular pathogenesis of several cancer entities, until recently there were no break-through discoveries on the events driving the carcinogenesis of Merkel cell carcinoma with the exception of the discovery by Feng et al,2 who demonstrated that Merkel cell carcinoma development in the majority of cases is preceded by the integration of genomic sequences of the hitherto unknown Merkel cell polyomavirus. Merkel cell polyomavirus has been found to be associated with only a subset of Merkel cell carcinoma, and investigations are underway to determine whether there are behavioural or clinical differences between virus-positive and virus-negative patients.3 Prognosis is related to stage and to the presence of lymph node metastases.4 However, in low-stage (stage I–II) Merkel cell carcinoma that is the vast majority of cases at presentation,4 no independent prognostic parameter is available.
A significant correlation between the expression of p63, a member of the p53 family, and the clinical course of Merkel cell carcinoma has been recently presented by us.5 The TP63 gene, mapped on chromosome 3q27–29, is expressed in basal and stem cells of the epidermis6, 7, 8 as well as in carcinomas from different sites. Eight different protein isoforms of p63 based on alternative splicing have been described.9, 10
The aim of the present study was to further evaluate in a large case series the immunohistochemical detection of p63 as a prognostic marker especially in low-stage (stage I–II) patients of Merkel cell carcinoma. Moreover, in all cases p63 expression by immunohistochemistry and molecular analysis of its variants were studied in parallel, to compare the two different procedures. Presence of Merkel cell polyomavirus was also investigated and related to the clinical outcome.
Materials and methods
Specimens
In all, 70 primary Merkel cell carcinomas from patients who underwent primary surgical treatment or post-surgical treatment at the Molinette Hospital, University of Turin (39 patients), during the period 1993–2010, at the Bellaria Hospital, University of Bologna (17 patients) during the period 1993–2010 and at the IRCC Candiolo (14 patients) during the period 2003–2010 were reviewed. For four patients (n. 2, 8, 20, 40), multiple distant metastases (lymph nodal and brain metastases) specimens were also available. Institutional Review Board permission was obtained for the study.
The following selection criteria were used: lesions primary of the skin, patients not previously treated for any other neuroendocrine malignancies or other primary skin tumours, availability of clinicopathological and follow-up data and of pathological material (slides and paraffin blocks). A total of 30 of the present cases had been included in a previous study.5
All patients were immunocompetent Caucasian individuals who had been surgically treated between 1993 and 2010.
Data regarding age, sex, presentation, gross pathology and follow-up were obtained from the clinical charts or directly from the referring pathologist or clinician.
Tumours were staged according to the 7th edition of the AJCC Cancer Staging Manual.4, 11
Pathology samples included resection specimens. Haematoxylin and eosin-stained slides (H&E) were available for all cases. Immunohistochemical and molecular analyses were carried out on all Merkel cell carcinomas, while FISH analysis was feasible in 55 cases (79%) only, because of the limited quantity of the tissue in 15 cases.
Immunohistochemistry
Immunohistochemical analysis was performed using chromogranin A antibody (clone LK2H10 Ventana-Diapath, Tucson, AZ, USA), synaptophysin antibody (clone SP11 mono-rabbit, Neomarkers, Fremont, CA, USA), cytokeratin 20 antibody (clone KS20.8, Dakopatts, Glostrup, Denmark), thyroid transcription factor-1 antibody (clone 847G3/1, Ventana-Diapath), CDX-2 (clone EPR2764Y, Ventana-Diapath), p63 antibody (clone 4A4, Dako, Santa Barbara, CA, USA), p53 antibody (clone DO-7, Ventana-Diapath) and Ki67 antibody (clone MIB-1, Dako) on 4-μm thick tissue sections from representative blocks of primary and metastatic Merkel cell carcinoma from all cases.
For a semiquantitative assessment of the intensity of staining and of the percentage of p63-positive and p53-positive cells, the modified histochemical score (H-score) was used, as we previously reported.5 Cases were classified as positive (≥10 of the H-score) and negative (<10 of the H-score). The H-score was not applied for the Ki67, and the proliferative index was expressed as a percentage based on the count of Ki67-positive cells in 2000 tumour cells in areas of the highest immunostaining.12 The status of p63, p53 and Ki67 was assessed without knowledge of the clinical and pathological features of the cases or the clinical outcome.
Molecular Analysis
Tissue blocks were selected for DNA and RNA extraction after careful examination on H&E staining of corresponding sections to exclude contaminating necrotic debris and normal skin. Molecular genetic analyses were performed on four or five sections (according to the size of lesion) of 10 μm (for DNA extraction) or of 20 μm (for RNA extraction) from paraffin-embedded tissue blocks. Tumour area was microdissected manually with a sterile blade to select the neoplastic cells to avoid the ‘epithelial contamination’.
Merkel cell polyomavirus DNA analysis
DNA was extracted using the RecoverAll kit (Ambion, Austin, TX, USA) in accordance to the manufacturer's instruction. For the presence of viral DNA, a PCR reaction was performed using the FastStartTaq kit (Roche, Mannheim, Germany). Primers for Merkel cell polyomavirus typing were previously described and named MCVPS1.13
p63 isoforms analysis
RNA was extracted using the RecoverAll kit (Ambion) in accordance to the manufacturer's instruction. RNA concentration was measured using Quant-it RNA kit (Invitrogen, Carlsbad, CA, USA). The sample concentration was normalised to obtain a total quantity of RNA of about 500 ng for each sample. RT-PCR was performed using the Transcriptor High Fidelity cDNA Synthesis Sample Kit (Roche, Mannheim, Germany).
PCR primers (Table 1) were designed using Primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi), and were synthesised by Integrated DNA Technologies (Coralville, IA, USA). Positive controls tested on breast tissues as previously reported,9 for the primers of p63 isoforms, were available to verify if our primers were effective at amplifying the targeted isoforms of p63 when some were never detected (data not shown).
FISH analysis
FISH studies were performed using a self-made TP63-specific locus probe BAC 373I6 (3q28) on seven Tissue Macro Array made as previously reported titles and legends to figures.14, 15 Tissue macro arrays were prepared with approximately four spots from each significant areas of all cases. Each tissue macro array was FISH tested with self-made red-labelled p63-specific locus probe and green chromosome 3 alpha satellite control probe (Cytocell Tehcnologies, UK). Tissue macro array red and green spots were automatically acquired with Metafer software (MetaSystem scanning station, Zeiss MetaSystems Gbmh), equipped with an AxioImager epifluorescence microscope. The automatically acquired images of Merkel cell carcinoma cells were analysed, with Isis software. About 50/100 non-overlapping neoplastic nuclei, with two control green signals (2G), were analysed for the each case and thus valuated: 2G/2R normal nuclei, 2G/R>2 p63 gain, 2G/R<2 p63 loss.
Statistical Analysis
All data were analysed using StataSE statistical software (version 10.0; StataCorp LT, College Station, TX, USA). A level of P<0.05 was considered to be statistically significant. Univariate survival analysis was based on the Kaplan–Meier16 product-limit estimate of the overall survival distribution of the entire survival experience of the patients. We also looked at the disease-free survival. The difference in survival between the two groups was determined using the Wilcoxon or Mantel–Cox tests.17 The relative significance of each parameter on survival included in the univariate analysis, was estimated using the Cox proportional hazards regression model determined by the Wald test (Z value). The Cox proportional hazards regression model assumption was satisfied for all prognostic variables in our models.
Results
Clinical and Pathological Features
There were 42 (60%) men and 28 (40%) women. Age at initial diagnosis ranged from 53 to 94 years (mean 76).
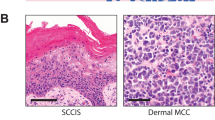
At presentation, all patients showed a single skin nodule, which was diagnosed as primitive Merkel cell carcinoma at histology (Figure 1a). The primary tumour sites were the extremities in 30 patients (43%), the head and neck in 24 patients (34%), the trunk in 5 patients (7%) and the buttocks in 11 patients (16%).
(a) Merkel cell carcinoma cells are predominantly arranged in small nests, show a non-cohesive growth pattern and demonstrate vesicular nuclei with well-outlined nuclear membranes. Nucleoli are small and chromatin is dispersed. (b) Neoplastic cells were found to be positive for p63 with variable intensity and percentage in 61.4% of Merkel cell carcinoma specimens. Epidermal basal cells serve as an internal positive control. (c) FISH with TP63 probe (BAC 373I6 in 3q28), red labelled (R) and chromosome 3 centromere, green labelled (G). The single case n. 29 has a gene gain with 3, 4, 5, 6 red signals/nucleo (mean 3R and 2.5G/nucleo) in more than 50% cell population.
The tumour size (greatest axis) ranged 0.3–12 cm (mean: 2.6 cm) and tumour thickness varied from 0.3 to 2.1 cm (mean: 1.1 cm). Tumour growth pattern was nodular in 22 (31%) and infiltrative in 48 (69%) Merkel cell carcinomas. Tumour cell size was larger than three lymphocytes in 46 of the 70 cases (66%) (large cells), and was equal or smaller than two lymphocytes in 24 cases (34%) (small cells). Nuclei were round–ovoid with stippled chromatin. Mitoses were ≥1 mitotic figure per mm2 in 52 of the 70 cases (74%). Vascular invasion was observed in 56 of 70 cases (80%). Tumour-infiltrating lymphocytes were identified in 41 (59%) Merkel cell carcinomas, of these 21 were brisk-type.11
Patients were stratified into the following categories: stage IA (25 cases, 36%), stage IIA (15 cases, 21%), stage IIIA (12 cases, 17%), stage IIIB (12 cases, 17%) and stage IV (6 cases, 9%).
Follow-up data were available for all 70 patients with a range of 2–142 months, mean of 31 months. In all, 29 cases presented with lymph node metastasis were associated with distant metastasis in five patients.
A total of 27 patients died of disease after a mean of 27 months (range: 2–142 months). In all, 12 patients were alive with extensive disease involving the soft tissues of leg and inguinal and abdominal lymph nodes after a mean of follow-up of 23 months (range: 5–98). Also 19 patients were alive without disease 42 months after surgical treatment (range: 5–106), and 12 patients died for other diseases (mean 30 months, range: 7–60) not related to Merkel cell carcinoma.
Kaplan–Meier survival analysis for clinicopathological variables demonstrated that only the stage was significantly associated with an adverse overall survival (P<0.012) and a decreased disease-free survival (P<0.001) (Tables 2 and 3).
Immunohistochemistry and Molecular Analysis
CK20 showed both the characteristic dot-like paranuclear cytoplasmic and membranous staining in the vast majority (>80%) of the cells from all cases. The same type of reactivity was found for neuroendocrine markers (chromogranin A and synaptophysin) in all cases, whereas thyroid transcription factor-1 and CDX-2 were negative in all cases.
In all, 43 of the 70 Merkel cell carcinomas (61%, Table 4) were found to be positive for p63 (Figure 1b) with variable intensity in primary Merkel cell carcinoma, and same results were obtained in the specimens from metastases (four lymph nodal and one brain metastases) (patients 2, 8, 20, 40). Positivity for p53 was present in 81% of the cases (57/70).
The proliferative labelling index (positivity for Ki67) ranged from 20 to 95% (mean of 51%, median of 48%) of positive nuclei in different cases. The cut-off value of Ki67 was 50% of positive nuclei and it was calculated using the mean value.
Kaplan–Meier survival analysis demonstrated that the expression of p63 was significantly associated with an adverse overall survival (P<0.0001) and a decreased disease-free survival (P<0.0001) when compared with the absence of p63 expression (Tables 2 and 3). In addition, survival analysis also demonstrated a significantly lower overall survival and disease-free survival with an increase in the stage of disease (P=0.012 and P=0.001, respectively) as well as tumours demonstrating a p53 positivity (P=0.032 and P=0.009) (Tables 2 and 3). Moreover, a decreased disease-free survival was observed with a proliferative index (Ki67) more than 50% (P<0.0001) (Table 3).
Multivariate Cox regression analysis estimated p63 expression to be a strong independent prognostic factor (P<0.001; hazard ratio: 7.26) followed by disease stage (P=0.008; hazard ratio: 1.82) in the 70 patients (Table 5).
In particular, Merkel cell carcinoma cases at low stage (stage I–II) that were positive for p63 by immunohistochemistry and RT-PCR (19 cases) demonstrated a more aggressive clinical course than those that were negative (P<0.0001) (Table 6). Indeed, patients affected with Merkel cell carcinoma positive for p63 showed an estimated 1-year overall survival rate of 90% either for stage I and stage II; 3-year overall survival rate of 79% for stage I and 57% for stage II; 5-year overall survival rate was 66% for stage I and 57% for stage II (Table 2).
At least one of the p63 isoforms by RT-PCR, in the primary Merkel cell carcinomas with a variable expression pattern of the isoforms was found in 42 out of 43 cases showing p63 positivity at immunohistochemistry (Table 4). In all, 27 out of 42 cases presented only one p63 isoform, while 13 cases showed two different isoforms and 2 cases four isoforms. The results were confirmed on control specimens (four lymph nodal and one brain metastases) from patients 2, 8, 20 and 40. In all, 2 out of these 25 Merkel cell carcinomas (patients 36 and 38) were not included in the analysis on the frequency of specific p63 isoforms because of the co-expression of two N-terminal and two C-terminal isoforms. In fact, the RNA extracted from paraffin-embedded tissues was regarded as very degraded, thus hampering to determine in these two samples which ΔN or TA isoform was associated to C-terminal isoforms. Specifically, TAp63α was the most frequently expressed isoform, being present in 26 of the 40 cases studied (65%), while the ΔNp63α was present in 14/40 cases (35%), ΔNp63β isoform in 5/40 cases (13%), TAp63β in 4/40 cases (10%) and TAp63γ in 4/40 (10%) (Figure 2). The remaining 27 cases, showing negativity for p63 at immunohistochemistry, did not display any p63 isoforms. No case expressed ΔNp63γ isoform and p63 isoforms with the same sequence as TAp63 and ΔNp63, but lacking exon 4 (Δ4TAp63 and ΔNp73L). Also in low stage (stage I–II), TAp63α and ΔNp63α taken together, were the most frequently expressed isoforms (42%), followed by co-expression of TAp63α/ΔNp63α isoforms in the same tumour (26%) by RT-PCR analysis.
Electrophoresis gel of two samples analysed for p63 isoform expression: (a) case 1 expressing ΔN-Beta isoform, (b) case 8 expressing TA-Alfa isoform. ΔNp73L and d4TAp63 are performed to double-check the expression of p63 isoforms lacking exon 4. B2M: beta-2-microglobulin; MW: molecular weight (range from 100 to 400 bp).
Kaplan–Meier survival analysis demonstrated that presence of at least one of the p63 isoforms was significantly associated with an adverse overall survival (P<0.0001) and with a decreased of disease-free survival (P<0.0001) (Tables 2 and 3).
The presence of Merkel cell polyomavirus DNA sequences was observed in 60/70 cases (86%) of Merkel cell carcinoma (Table 4), together with significant expression levels of transforming viral sequences (Figure 3).
In particular, the co-expression of p63 and Merkel cell polyomavirus in Merkel cell carcinoma cases was observed in 34 patients and all, but one (patient n. 29) had at least one of p63 isoforms by RT-PCR. The latter case has a slight gain of the TP63 gene by FISH (see below) but this data, by itself did not have prognostic significance (Tables 2 and 3).
FISH Results
FISH results are summarised in Table 4.
All samples of Merkel cell carcinoma were analysed by FISH to verify if TP63 gain/amplification would be involved in p63 expression in Merkel cell carcinoma. Only 55 samples, of the 70 analysed, had readable FISH signals: 54 showed normal pattern, 2 green/2 red, in almost all analysed nuclei, only 1 case (patient 29) had a slight gain of the gene, with a mean of 2.5 green and 3 red per nuclei in more that 50% of cell population (Figure 1c). The latter case showed p63 positivity by immunohistochemistry but RT-PCR analysis failed to find any p63 isoform.
Discussion
In the present immunohistochemical and molecular study for p63, the two procedures showed the same accuracy to assess p63 expression in Merkel cell carcinomas. With regard to the TP63 gene status, the FISH analysis has not pointed out any polisomy/amplification, except in a case in which a TP63 gene gain has been noticed in most nuclei of the neoplastic cells. This patient, who showed p63 expression by immunohistochemistry but failed to display any p63 isoforms at RT-PCR level, died of disease with multiple distant metastases after 52 months from surgery. These data suggest that TP63 gene gain is a rare event and it seems not primary involved in p63 expression in Merkel cell carcinoma cells. In the 70 cases, multivariate Cox regression analysis assessed p63 expression to be a strong independent prognostic factor (P<0.001) followed by disease stage (P=0.008). Without unduly dismissing the importance of staging, it should be remarked that the real clinical dilemma is related to localised stage I and II Merkel cell carcinomas, which represent the vast majority at first presentation4 and where no predictor is available to identify the large number of cases behaving aggressively4 and to plan therapy accordingly. Indeed, we wish to stress that the utility and importance of data from the current study is that p63 expression was found to be strongly associated with a worse prognosis in patients with low-stage Merkel cell carcinoma and represents a new independent marker of clinical evolution in these patients (P<0.0001) (Figure 4). In our case series 48% (19/40 cases) of patients with either stage I or II disease showed, at the time of diagnosis, p63 expression by immunohistochemistry and we observed that, also in low-stage tumours, TAp63α and ΔNp63α taken together, were the most frequently expressed isoforms (42%), followed by the co-expression of TAp63α/ΔNp63α isoforms in the same tumour (26%). All but one of patients that died of disease or were alive with disease were p63 positive, while 95% (20/21 cases) of patients that had no evidence of disease at follow-up (11 cases) or died of other cause (9 cases), respectively, were p63 negative. The present comparative and parallel use of different procedures in a large case series validates the immunohistochemical approach for detection of p63 expression as a reliable prognostic marker detecting all p63 variants in our series of Merkel cell carcinoma. We feel entitled to suggest that beginning from low stage of disease an anomalous regulation of the expression of TAp63α and ΔNp63α isoforms of p63 might cause a characteristic phenotype switch towards a more aggressive Merkel cell carcinoma subgroup. Merkel cell carcinoma is a neuroendocrine carcinoma, expressing neuroendocrine markers such as chromogranin and synaptophisin. However, a fraction of those tumours express p63, possibly denoting both a switch towards a stem cell component (expression of ΔNp63α) and an acceleration in tumour development and progression (expression of TAp63α).6
Merkel cell carcinoma is a highly aggressive tumour, with 33% mortality within 3 years from diagnosis,18 and its clinical staging is based on a 4-tiered system.4 In our case series, at the time of diagnosis, over half (57%, 40/70 cases) of the patients presented with either stage I or II disease. In all, 25% (10/40 cases) of patients showing stage I–II died of disease after a mean of 34 months and 13% (5/40 cases) were alive with disease after a mean of 20 months.
A polyomavirus was seen to be associated to 80% of Merkel cell carcinoma.2 The virus, provisionally designed Merkel cell polyomavirus, was indicated to be the aetiological agent of Merkel cell carcinoma.19, 20, 21, 22, 23 Accordingly, presence of Merkel cell polyomavirus DNA sequences was observed in 86% of our case series, together with significant expression levels of transforming viral sequences.
Based on our data, we confirm that p63, possibly denoting a switch towards a stem cell phenotype, might be the most consistent predictor of survival of Merkel cell carcinoma, and we suggest that the oncogenic potential of p63 may reside in activating p53-mediated oncogenic pathways carrying an aggressive and lethal behaviour. Interestingly, the observed relationship of the stem cell phenotype of Merkel cell carcinoma with a more aggressive clinical course is similar to that reported in other lineage-unrelated skin entities. In fact, progression to malignant melanoma has been shown to be associated with expression of stem cell markers (CD166, CD133 and nestin),24 as well as in epithelial lesions the expression of stem cell markers (cytokeratin 15, cytokeratin 19 and p63) has been noted to match with grading and development of in situ and invasive malignancies.25
References
Kohler S, Kerl H . Merkel cell carcinoma. In: LeBoit E, Burg G, Weedon D, Sarasin A (eds). World Health Organization Classification of Tumours. Pathology and Genetics of Skin Tumors. IARC Press: Lyon, 2006, pp 272–273.
Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008;319:1096–1100.
The Rockwell Merkel Cell Carcinoma Group. Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis, and clinical management. J Clin Oncol 2009;27:4021–4026.
Lemos BD, Storer BE, Iyer JC, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol 2010;63:751–761.
Asioli S, Righi A, Volante M, et al. p63 expression as a new prognostic marker in Merkel cell carcinoma. Cancer 2007;110:640–647.
Mills AA, Zheng B, Wang XJ, et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 1999;398:708–713.
Yang A, Schweitzer R, Sun D, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 1999;398:714–718.
Yang A, Kaghad M, Wang Y, et al. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell 1998;2:305–316.
de Biase D, Morandi L, Degli Esposti R, et al. p63 short isoforms are found in invasive carcinomas only and not in benign breast conditions. Virchows Arch 2010;456:395–401.
Foschini MP, Gaiba A, Cocchi R, et al. Pattern of p63 expression in squamous cell carcinoma of the oral cavity. Virchows Arch 2004;444:332–339.
Rao P, Balzer BL, Lemos BD, et al. Protocol for the examination of specimens from patients with merkel cell carcinoma of the skin. Arch Pathol Lab Med 2010;134:341–344.
Scarpa A, Mantovani W, Capelli P, et al. Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol 2010;23:824–833.
Duncavage EJ, Zehnbauer BA, Pfeifer JD . Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol 2009;22:516–521.
Lambros MB, Simpson PT, Jones C, et al. Unlocking pathology archives for molecular genetic studies: a reliable method to generate probes for chromogenic and fluorescent in situ hybridization. Lab Invest 2006;86:398–408.
Castellano I, Sapino A, Arisio R, et al. Fluorescent in situ hybridization as a screening test for HER2 amplification in G2 and G3 breast cancers of lobular and ductal histotype and metastases. Oncol Rep 2008;19:1271–1275.
Kaplan E, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481.
Cox D . Regressions models and life table. JR Stat Soc 1972;34:187–220.
Allen PJ, Browne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol 2005;23:2300–2309.
Kassem A, Schopflin A, Diaz C, et al. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res 2008;68:5009–5013.
Garneski KM, Warcola AH, Feng Q, et al. Merkel cell polyomavirus is more frequently present in North American than Australian Merkel cell carcinoma tumours. J Invest Dermatol 2009;129:246–248.
Becker JC, Houben R, Ugurel S . MC polyomavirus is frequently present in Merkel cell carcinoma of European patients. J Invest Dermatol 2009;129:248–250.
Shuda M, Feng H, Kwun HJ, et al. T antigen mutations are a human tumour-specific signature for Merkel cell polyomavirus. Proc Natl Acad Sci USA 2008;105:16272–16277.
Schowalter RM, Pastrana DV, Pumphrey KA, et al. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe 2010;7:509–515.
Klein WM, Wu BP, Zhao S, et al. Increased expression of stem cell markers in malignant melanoma. Mod Pathol 2007;20:102–107.
Abbas O, Richards JE, Yaar R, et al. Stem cell markers (cytokeratin 15, cytokeratin 19 and p63) in in situ and invasive cutaneous epithelial lesions. Mod Pathol 2011;24:90–97.
Acknowledgements
We would like to thank Drs Roberto Vigliani, Giuseppe Forte, Giovanni Cera, Flavia Botto Micca and Renato Coda for kindly supplying cases. Work supported by grants of the Italian Ministry of University (ex-60%) and Ricerca Sanitaria Finalizzata Piedmont Region (to SA). Righi A is part of and funded by the PhD programme ‘Scienze Biomediche ed Oncologia Umana: Tecniche avanzate di localizzazione dei tumori umani’, University of Turin de Biase D is founded by C.I.R.C. (Centro Interdipartimentale di Ricerche sul Cancro ‘G.Prodi’, University of Bologna).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Modern Pathology website
Supplementary information
Rights and permissions
About this article
Cite this article
Asioli, S., Righi, A., de Biase, D. et al. Expression of p63 is the sole independent marker of aggressiveness in localised (stage I–II) Merkel cell carcinomas. Mod Pathol 24, 1451–1461 (2011). https://doi.org/10.1038/modpathol.2011.100
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2011.100
Keywords
This article is cited by
-
Prognostic Impact of MCPyV and TIL Subtyping in Merkel Cell Carcinoma: Evidence from a Large European Cohort of 95 Patients
Endocrine Pathology (2020)
-
PD-1 (PDCD1) promoter methylation in Merkel cell carcinoma: prognostic relevance and relationship with clinico-pathological parameters
Modern Pathology (2019)
-
Update on Merkel Cell Carcinoma
Head and Neck Pathology (2018)
-
Merkel Cell Carcinoma: Current Issues Regarding Diagnosis, Management, and Emerging Treatment Strategies
American Journal of Clinical Dermatology (2016)