Abstract
Identification of specific somatic gene alterations is crucial for the insight into the development, progression, and clinical behavior of individual cancer types. The recently discovered recurrent ERG rearrangement in prostate cancer might represent a prostate cancer-specific alteration that has not been systematically assessed in tumors other than prostate cancer. Aim of this study was to assess, whether the ERG rearrangement and the distinct deletion site between TMPRSS2 and ERG, both predominantly resulting in a TMPRSS2–ERG fusion, occur in tumors other than prostate cancer. We assessed 54 different tumor types (2942 samples in total) for their ERG rearrangement status by fluorescence in situ hybridization (FISH). To calibrate, we analyzed 285 prostate cancer samples for the ERG rearrangement frequency. Additionally, we interrogated a high-resolution single nucleotide polymorphism (SNP) data set across 3131 cancer specimens (26 tumor types) for copy number alterations. None of the 54 different tumor types assessed by FISH harbored an ERG rearrangement, whereas the prostate cancer samples revealed an ERG rearrangement in 49.5% of cases. Furthermore, within the 26 tumor types assessed for copy number alterations by SNP, the distinct deletion site between TMPRSS2 and ERG (21q22.2–3) was detectable exclusively in prostate cancer. Although Ewing's sarcoma and AML have known rearrangements rarely involving ERG, we hypothesize that the ERG rearrangement as well as the distinct deletion site on 21q22.2–3 between TMPRSS2 and ERG are prostate-cancer-specific genomic alterations. These observations provide further insight into the oncogenesis of prostate cancer and might be critical for the development of ERG rearrangement assessment as a clinical tool.
Similar content being viewed by others
Main
Like other cancers, prostate cancer is driven by the acquisition of somatic genetic alterations resulting in oncogenesis. Among these, recurrent gene translocations have been supposed to be specific for hematological and mesenchymal malignancies (sarcomas) until recently.1 Translocations have only been observed in rare subtypes of common epithelial malignancies (carcinomas) such as breast, thyroid, and renal.2, 3 The recent discovery that the majority of prostate cancers harbor recurrent gene rearrangements suggests that translocations may occur more commonly than previously assumed in epithelial cancers.4 The nature of recurrent gene rearrangements in prostate cancer involves androgen-regulated 5′ partners (eg, TMPRSS2, SLC45A3, and NDRG1) and ETS genes (eg, ETV1, ETV4, and ETV5), most commonly resulting in the TMPRSS2–ERG fusion.5 Within this distinct subgroup of prostate cancer, the TMPRSS2–ERG fusion occurs through a deletion of the genomic material spanning from TMPRSS2 to ERG in the majority of these cases.6, 7 It is intriguing to note that ETS genes have been detected earlier in translocations in Ewing's sarcoma, AML, and breast cancer. In these tumors the most frequent ETS genes involved differ from prostate cancer. For example, in Ewing's sarcoma, the ETS gene FLI1 is fused to EWS most commonly, whereas EWS–ERG fusions occur rarely.8 Given the high frequency of prostate cancers harboring ERG rearrangement, we undertook a large survey of common tumors to exclude the possibility that any of these harbor the ERG rearrangement. Additionally, to identify prostate-cancer-specific somatic copy number alterations, we interrogated a recently published and publicly available high-resolution data set of somatic copy number alterations across 3131 cancer specimens, derived from 26 different histological types.9
Materials and methods
Samples
We assessed 54 different tumor types (2942 tumor samples in total) for their ERG rearrangement status using an ERG break-apart fluorescence in situ hybridization (FISH) assay as described earlier.4, 6, 10 This assay can differentiate between the structural ERG rearrangement (ie, ERG rearrangement through insertion) and the distinct deletion on 21q22.2–3 associated with the ERG rearrangement (ie, ERG rearrangement through deletion), both most commonly resulting in a fusion with TMPRSS2.6 In all, 2261 cases were assessable. These included 131 breast carcinomas, 36 colon carcinomas, 111 colon adenomas, 120 non-small cell lung carcinomas, 32 small cell lung cancers, 94 urinary bladder carcinomas, 85 kidney carcinomas, 77 thyroid carcinomas, 74 ovarian carcinomas, 68 endometrial carcinomas, 63 non-Hodgkin lymphomas, 60 malignant melanomas, 59 basal cell carcinomas, 58 hepatocellular carcinomas, 48 stomach carcinomas, 45 seminomas, 44 Schwann cell tumors, 44 non-seminomatous testicular carcinomas, 42 uterine leiomyomata, 41 pleomorphic adenomas of the salivary glands, 41 leiomyosarcomas, 40 oral cavity carcinomas, 40 meningiomas, 40 astrocytomas, 38 renal oncocytomas, 38 glioblastomas, 37 pancreatic carcinomas, 36 esophagus carcinomas, 36 Hodgkin lymphomas, 34 parathyroideal adenomas, 34 thymomas, 33 gallbladder carcinomas, 33 nevi, 33 carcinoid tumors, 32 neurofibromas, 31 laryngeal carcinomas, 29 thyroid adenomas, 29 malignant fibrous histiocytomas, 27 hemangiomas, 25 squamous cell skin cancers, 25 salivary gland cylindromas, 24 benign histiocytomas, 21 pheochromocytomas, 21 tendon sheath giant cell tumors, 21 liposarcomas, 19 mesotheliomas, 19 vulvar carcinomas, 19 oligodendrogliomas, 18 salivary gland adenolymphomas, 15 duodenal carcinomas, 13 benign skin tumors (NOS), 12 adrenal adenomas, 8 Kaposi's sarcomas, and 8 paragangliomas. All patients were diagnosed at the University Hospital of Basel, Switzerland. Of these samples, six tissue micro arrays were constructed with one core per case with a diameter of 0.6 mm.
To calibrate the frequency of ERG rearrangement prostate cancer, we assessed a well-defined cohort using a break-apart FISH assay. The cohort contains tumor material from 109 consecutive partially PSA-screened patients who underwent prostatectomy. Two cores were taken from the index tumor focus from the peripheral zone of each patient sample. Patients were treated at the University Hospital of Tuebingen.
Tissue Micro Array Construction
Formalin-fixed paraffin-embedded prostate cancer specimen were cut in 4 μm thick sections, mounted on slides, and stained with hematoxylin and eosin. Subsequently, the cancer region was marked. The cores, each 0.6 mm in diameter, were taken from the corresponding donor block and placed into a tissue micro array recipient block using a semiautomatic tissue arrayer (Beecher Instruments, Sun Prairie, WI, USA); 4 μm thick tissue sections were placed onto superfrost slides.
Fluorescence In Situ Hybridization
We used a FISH assay to detect the ERG rearrangement at the chromosomal level on formalin-fixed paraffin-embedded specimen. Hence, we performed a split-signal-approach, with two probes spanning the ERG locus as described earlier.4, 6 Deparaffinized sections were pretreated with a 100 mM Tris and 50 mM EDTA solution at 92.8°C for 15 min and digested with Digest-All III (dilution 1:2) at 37°C for 22 min; ERG FISH probes were denatured at 73°C for 5 min and immediately placed on ice. Subsequently, the tissue sections and ERG FISH probes were co-denatured at 94°C for 3 min and hybridized overnight at 37°C. We used BAC clones RP11-24A11 for centromeric labeling with biotin and RP11-372O17 for telomeric labeling with digoxigenin. Posthybridization washing was performed with 2 × SSC at 75°C for 7 min, and the fluorescence detection was carried out using streptavidin-Alexa-594 conjugates (dilution 1:200) and anti-digoxigenin-FITC (dilution 1:200). Slides were then counterstained with 4′,6-diamidin-2′ phenylindoldihydrochlorid (DAPI) and mounted.
The samples were analyzed under an × 63 oil immersion objective using a fluorescence microscope (Zeiss, Jena, Germany) equipped with appropriate filters, a charge-coupled device camera, and the FISH imaging and capturing software Metafer 4 (Metasystems, Altlussheim, Germany). All cases were independently assessed by three experienced evaluators (MB, VS, and SP) At least 100 nuclei per case were evaluated.
This FISH assay allows for ERG rearrangement status (ie rearrangement versus no rearrangement of ERG) assessment. The assay is also capable of differentiating between two different mechanisms of ERG rearrangement.6 These two mechanisms are ERG rearrangement through insertion and ERG rearrangement through deletion of DNA between TMPRSS2 and ERG loci (interstitial deletion). A nucleus without an ERG rearrangement shows two pairs of juxtaposed red and green signals (mostly forming two yellow signals). A nucleus with an ERG rearrangement through insertion shows the split of a signal pair resulting in a single red and single green signal for the rearranged ERG allele and a still juxtaposed (yellow) signal pair for the non-rearranged ERG allele in each nucleus. A nucleus with an ERG rearrangement through deletion shows one juxtaposed red-green signal pair (yellow) for the non-rearranged allele and a single red signal for the allele involved in the rearrangement.
Analysis of Somatic Copy Number Alterations
Beroukhim et al9 published a publicly available high-resolution single nucleotide polymorphism (SNP) data set of somatic copy number profiles across 3131 cancer specimens. We obtained average copy number profiles from 26 cancer types, including prostate cancer, each represented from at least 20 and up to 734 cancer specimens. Seventeen cancer types are represented by at least 40 specimens. In all, 2520 profiles were obtained from tissue specimens and 611 were obtained from cancer cell lines or short-term cultures. All copy number estimates were obtained using an SNP array (Affymetrix 250K Sty). Signal intensities from each cancer specimen were compared to SNP data obtained from 1480 specimens of normal tissue to identify regions of somatically generated copy number alterations. The raw data of this study were obtained from http://www.broadinstitute.org/tumorscape.
Results
By assessing 2942 tumor samples of 54 different common tumors for their ERG-rearrangement status using our ERG break-apart FISH assay, we could show that none of the 2261 assessable epithelial and non-epithelial tumors other than prostate cancer harbored a rearrangement of the ERG locus. For a detailed break down of the individual tumor entities assessed for the ERG rearrangement, see Table 1.
For calibration of the ERG rearrangement frequency we assessed a well-defined prostate cancer cohort by the same ERG break-apart FISH assay. We observed a frequency of the ERG rearrangement in 54/109 (49.5%) of cases in the partially PSA-screened prostatectomy cohort. Of these, 40/54 (74.1%) harbored the ERG rearrangement through deletion, whereas 14/54 (25.9%) harbored the ERG rearrangement through insertion.
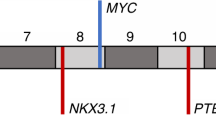
The ERG break-apart FISH assay is capable of differentiating between the structural ERG rearrangement (ie ERG rearrangement through insertion) and the distinct numerical 21q22.2–3 alteration associated with the ERG rearrangement (ie ERG rearrangement through deletion). As indicated above, the ERG rearrangement through deletion is the prevalent mechanism in our prostate cancer cohort (ie 40 cases with ERG rearrangement through deletion versus 14 cases with ERG rearrangement through insertion). To determine whether the distinct deletion site between TMPRSS2 and ERG is specific to prostate cancer, we examined copy number profiles from 3131 cancers across multiple cancer types.9 Interestingly, we only observed the distinct deletion site between TMPRSS2 and ERG in prostate cancer but in none of the other interrogated tumor types. A summary of these data can be seen in Figure 1, which shows the average copy number profiles on chromosome 21q for each of the 26 cancer types represented by at least 20 specimens.
Average copy number changes across chromosome 21q for 26 different cancer types. Each cancer type (arranged along the x axis) represents average log2 ratios from SNP array data obtained from at least 20 and up to 734 cancer specimens. Only prostate cancer exhibits characteristic deletions between ERG and TMPRSS2.
In independent tumor samples, a significant subset of tumor entities was both assessed for the ERG rearrangement status by the break-apart FISH assay and for copy number alterations by SNP analysis. As expected, we found losses and gains of the FISH signals at different levels in subsets of the tumors highly corresponding to the copy number assessments by SNP analysis (data not shown). To emphasize, FISH signal patterns specific to ERG rearrangement through deletion or ERG rearrangement through insertion only appeared in prostate cancer samples.
Discussion
Important implications for the diagnosis, prediction, prognosis, and development of therapeutic targets in cancers can be obtained from systemic efforts to identify somatic genetic alterations. Of these, cancer-type-specific alterations are most promising. Surprisingly, the recurrent ERG gene rearrangement was recently discovered in the majority of prostate cancers.5, 6, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 By assessing 2295 samples comprising a broad spectrum of tumor entities, we were the first to prove that the ERG rearrangement does not occur in other common epithelial and non-epithelial tumors. Although Ewing's sarcoma and AML have known rearrangements sporadically involving ERG (ie EWS-ERG and FUS-ERG, respectively), the current finding supports the hypothesis that the mentioned mechanisms of ERG rearrangement are specific to prostate cancer. These findings are essential for the application of ERG rearrangement as a clinical marker and might have further implications for the development of targeted therapies. In addition, by interrogating the Beroukhim data set of somatic copy number profiles across 3131 cancer specimens, we found that the distinct deletion site on 21q22.2–3 spanning from TMPRSS2 to ERG is a copy number alteration specific to prostate cancer and is not shared by other human cancers.9 Furthermore, we could verify that the distinct numerical 21q22.2–3 alteration associated with the ERG rearrangement (ie ERG rearrangement through deletion) is the prevalent mechanism resulting in the TMPRSS2–ERG gene fusion in prostate cancer.6, 7 It is of particular interest that this specific deletion most frequently results in the TMPRSS2–ERG fusion. Beroukhim et al compared the significant somatic copy number alterations in each of the individual cancer types to the remaining samples and found that the majority of somatic copy number alterations is shared by different cancers. This indicates that the major genomic alterations on 21q22.2–3, ie the ERG rearrangement and the distinct deletion site spanning from TMPRSS2 to ERG, both resulting in the TMPRSS2–ERG gene fusion, are specific to prostate cancer. Of note, in our previous study, we could identify significantly downregulated genes with tumor suppressor potential located in the area of the distinct deletion site on 21q22.2–3.6 The additional loss of these genes might have important biological and clinical effects specific to prostate cancer.
Until now, the reason for the occurrence of cancer-type-specific gene fusions is largely unknown. Recently, Mani et al23 could provide evidence that androgen stimulation causes a physical approximation of TMPRSS2 and ERG in androgen-sensitive prostate cancer cell lines. Subsequent irradiation—representing a DNA-double-strand-breaking event—resulted in accumulated TMPRSS2–ERG fusions. As prostate cancer is the only androgen-sensitive malignancy, these findings could help to better understand why the TMPRSS2–ERG gene fusion is a prostate-cancer-specific genomic alteration. In this context it is worth mentioning that complex interactions between chromosomes and folding patterns represent a broad field that is poorly elucidated. Lieberman-Aiden et al24 described a method that allows identification and visualization of chromatin interactions across a whole genome. Using this technique, complex relationships between chromatin structure, gene activity, and the functional state of the cell might be assessed in cancers with specific genomic alterations. Thus, new aspects of the genesis of genomic alterations might be provided.
In summary, we were the first to assess a large collection of common epithelial and non-epithelial tumors and found that the distinct genomic alterations on 21q22.2–3, ie the ERG rearrangement and the deletion site spanning from TMPRSS2 to ERG, both resulting in the TMPRSS2–ERG gene fusion, are specific to prostate cancer and do not occur in any other common tumor. Despite the broad spectrum of tumors and the large number of cases for each tumor entity we evaluated, a sporadic appearance of the ERG rearrangement in rare tumors not assessed by us or in an extremely low frequency in the assessed tumor entities cannot be excluded.
We believe that a genetic alteration, which is specific to malignant cells, might be targeted by modern therapies. In recent studies by Wang et al25 and Sun et al26, a knock down of TMPRSS–ERG in fusion-positive cells has been shown to inhibit tumor growth in xenograft assays. Additionally, as the ERG rearrangement is assumed to drive prostate cancer development through downstream target genes, downstream genes might be promising candidates for new therapeutic strategies.5
Furthermore, the ERG rearrangement might gain relevance in diagnostic usage as this alteration can be detected in urine samples and biopsies. Confirming prostate cancer by the detection of the prostate-cancer-specific ERG rearrangement can be helpful in malignancies of unknown primary such as bone metastasis or small cell cancers of unclear origin.27 In these cases, early therapy can dramatically slow down the progress of the disease. The diagnostic step forward is already concrete, therapeutic impact in terms of rational therapy has to be investigated.
References
Mitelman F, Johansson B, Mertens F . The impact of translocations and gene fusions on cancer causation. Nat Rev Cancer 2007;7:233–245.
Teixeira MR . Recurrent fusion oncogenes in carcinomas. Crit Rev Oncog 2006;12:257–271.
Letessier A, Ginestier C, Charafe-Jauffret E, et al. ETV6 gene rearrangements in invasive breast carcinoma. Genes Chromosomes Cancer 2005;44:103–108.
Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005;310:644–648.
Tomlins SA, Bjartell A, Chinnaiyan AM, et al. ETS gene fusions in prostate cancer: from discovery to daily clinical practice. Eur Urol 2009;56:275–286.
Perner S, Demichelis F, Beroukhim R, et al. TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res 2006;66:8337–8341.
Liu W, Chang B, Sauvageot J, et al. Comprehensive assessment of DNA copy number alterations in human prostate cancers using Affymetrix 100K SNP mapping array. Genes Chromosomes Cancer 2006;45:1018–1032.
Burchill SA . Molecular abnormalities in Ewing's sarcoma. Expert Rev Anticancer Ther 2008;8:1675–1687.
Beroukhim R, Mermel CH, Porter D, et al. The landscape of somatic copy-number alteration across human cancers. Nature 2010;463:899–905.
Perner S, Wagner PL, Demichelis F, et al. EML4-ALK fusion lung cancer: a rare acquired event. Neoplasia 2008;10:298–302.
Clark J, Attard G, Jhavar S, et al. Complex patterns of ETS gene alteration arise during cancer development in the human prostate. Oncogene 2008;27:1993–2003.
Kumar-Sinha C, Tomlins SA, Chinnaiyan AM . Recurrent gene fusions in prostate cancer. Nat Rev Cancer 2008;8:497–511.
Iljin K, Wolf M, Edgren H, et al. TMPRSS2 fusions with oncogenic ETS factors in prostate cancer involve unbalanced genomic rearrangements and are associated with HDAC1 and epigenetic reprogramming. Cancer Res 2006;66:10242–10246.
Mehra R, Han B, Tomlins SA, et al. Heterogeneity of TMPRSS2 gene rearrangements in multifocal prostate adenocarcinoma: molecular evidence for an independent group of diseases. Cancer Res 2007;67:7991–7995.
Cerveira N, Ribeiro FR, Peixoto A, et al. TMPRSS2-ERG gene fusion causing ERG overexpression precedes chromosome copy number changes in prostate carcinomas and paired HGPIN lesions. Neoplasia 2006;8:826–832.
Nam RK, Sugar L, Yang W, et al. Expression of the TMPRSS2:ERG fusion gene predicts cancer recurrence after surgery for localised prostate cancer. Br J Cancer 2007;97:1690–1695.
Perner S, Mosquera JM, Demichelis F, et al. TMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol 2007;31:882–888.
Soller MJ, Isaksson M, Elfving P, et al. Confirmation of the high frequency of the TMPRSS2/ERG fusion gene in prostate cancer. Genes Chromosomes Cancer 2006;45:717–719.
Wang J, Cai Y, Ren C, et al. Expression of variant TMPRSS2/ERG fusion messenger RNAs is associated with aggressive prostate cancer. Cancer Res 2006;66:8347–8351.
Yoshimoto M, Joshua AM, Chilton-Macneill S, et al. Three-color FISH analysis of TMPRSS2/ERG fusions in prostate cancer indicates that genomic microdeletion of chromosome 21 is associated with rearrangement. Neoplasia 2006;8:465–469.
Mosquera JM, Mehra R, Regan MM, et al. Prevalence of TMPRSS2-ERG fusion prostate cancer among men undergoing prostate biopsy in the United States. Clin Cancer Res 2009;15:4706–4711.
Clark JP, Cooper CS . ETS gene fusions in prostate cancer. Nat Rev Urol 2009;6:429–439.
Mani RS, Tomlins SA, Callahan K, et al. Induced chromosomal proximity and gene fusions in prostate cancer. Science 2009;326:1230.
Lieberman-Aiden E, van Berkum NL, Williams L, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 2009;326:289–293.
Wang J, Cai Y, Yu W, et al. Pleiotropic biological activities of alternatively spliced TMPRSS2/ERG fusion gene transcripts. Cancer Res 2008;68:8516–8524.
Sun C, Dobi A, Mohamed A, et al. TMPRSS2-ERG fusion, a common genomic alteration in prostate cancer activates C-MYC and abrogates prostate epithelial differentiation. Oncogene 2008;27:5348–5353.
Scheble V, Braun M, Wilbertz T, et al. ERG rearrangement in small cell lung and prostate cancer. Histopathology 2010: in press.
Acknowledgements
This work was supported by a grant of the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG, Emmy-Noether-Program, PE1179/2-1) and the University Hospital of Tuebingen (fortuene Program, No. 1809-1-0) to SP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The Brigham and Women's Hospital and the University of Michigan have filed a patent on ETS gene rearrangements in prostate cancer, on which SP and MAR are co-inventors and the diagnostic field of use has been licensed to GenProbe. GenProbe has neither played a role in the design and conduct of the study, nor in the collection, analysis, or interpretation of the data and no involvement in the preparation, review, or approval of the manuscript.
Rights and permissions
About this article
Cite this article
Scheble, V., Braun, M., Beroukhim, R. et al. ERG rearrangement is specific to prostate cancer and does not occur in any other common tumor. Mod Pathol 23, 1061–1067 (2010). https://doi.org/10.1038/modpathol.2010.87
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2010.87
Keywords
This article is cited by
-
Deglycosylation of pathological specimens alters performance of diagnostic PDL1 antibodies
Virchows Archiv (2022)
-
Ecotropic viral integration site 1, a novel oncogene in prostate cancer
Oncogene (2017)
-
Comparison of different prostatic markers in lymph node and distant metastases of prostate cancer
Modern Pathology (2015)
-
Das neuroendokrine Prostatakarzinom
Der Urologe (2015)
-
Staurosporine analogs promote distinct patterns of process outgrowth and polyploidy in small cell lung carcinoma cells
Tumor Biology (2015)