Abstract
On the basis of recent clinical studies, some urologic oncologists do not offer bladder-sparing therapy for patients diagnosed with micropapillary carcinoma of the urinary bladder, even in the setting superficially invasive disease. Unfortunately, the distinction of invasive micropapillary carcinoma from typical invasive urothelial carcinoma with prominent retraction artifact may be difficult in some cases. In this study, we compared the immunophenotype of invasive micropapillary carcinoma to invasive urothelial carcinoma with retraction artifact using antibodies previously reported as specific for micropapillary carcinoma. Immunohistochemical staining was performed on 24 invasive micropapillary carcinomas of the urinary tract and 24 case controls of invasive urothelial carcinoma with retraction artifact using monoclonal antibodies MUC1, CA125, and Her2Neu. The staining extent and intensity for MUC1 and CA125 were scored on one representative section per case. Immunostaining for Her2Neu was scored based on the 2007 CAP/ASCO guidelines for breast carcinoma. Basal (‘reverse-apical’) MUC1 staining was identified in 23 of the 24 (96%) invasive micropapillary carcinomas and in 15 of the 24 (63%) invasive urothelial carcinomas with retraction artifact (P=0.0102). Membranous reactivity with CA125 was seen in 8 of the 24 (33%) invasive micropapillary carcinomas and in 3 of the 24 (13%) invasive urothelial carcinomas with retraction artifact (P=0.1681). Positive (3+) membranous Her2Neu staining was present in 6 of 24 (25%) invasive micropapillary carcinomas and in 2 of the 24 (8%) invasive urothelial carcinomas with retraction artifact (P=0.2448). The specificity for invasive micropapillary carcinoma vs invasive urothelial carcinoma with retraction artifact using antibodies MUC1, CA125, and Her2Neu was 37, 87, and 92%, respectively. Invasive micropapillary carcinoma more commonly showed immunoreactivity for MUC1, CA125, and Her2Neu compared to invasive urothelial carcinoma with retraction artifact, but only MUC1 reached statistical significance. The lack of specificity of these evaluated markers for invasive micropapillary carcinoma limits their utility in the distinction from invasive urothelial carcinoma with retraction artifact, especially given the potentially significant therapeutic implications.
Similar content being viewed by others
Main
Since the initial description of invasive micropapillary carcinoma of the urinary bladder by Amin et al in 1994,1 micropapillary carcinoma has been well characterized as a unique histologic variant in several other organs including the breast, lung, salivary gland, and colon with a propensity for high-stage disease and poor prognosis compared to conventional carcinomas arising in the same organ.2, 3, 4, 5, 6 Moreover, micropapillary carcinoma has been described within other sites along the urinary tract including the ureter and renal pelvis.7, 8, 9, 10, 11, 12 The distinction of invasive micropapillary carcinoma from typical invasive urothelial carcinoma with prominent retraction artifact may be difficult in a subset of cases. Several studies and case reports have examined the immunophenotype of invasive micropapillary carcinoma with or without comparison to usual invasive urothelial carcinoma and have suggested MUC1, CA125, and Her2Neu as sensitive and specific markers for invasive micropapillary carcinoma.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 However, a formal comparison of the immunoprofile of invasive micropapillary carcinoma and typical invasive urothelial carcinoma with prominent retraction artifact has not been previously performed. Given recent clinical studies suggesting aggressive treatment strategies for patients with micropapillary urothelial carcinoma,21, 22 the aim of this study was to examine the diagnostic utility of MUC1, CA125, and Her2Neu immunohistochemistry in the distinction of invasive micropapillary carcinoma from typical invasive urothelial carcinoma with stromal retraction artifact.
Materials and methods
Twenty-four invasive micropapillary carcinomas of the urinary tract and an equal number of typical invasive urothelial carcinomas with prominent retraction artifact, matched for age, sex, specimen type, and stage (best possible match), were retrieved from the pathology archives of Stanford University Medical Center, Veterans Affairs Palo Alto Health Care System, and the University of Arkansas for Medical Sciences. Each case was reviewed by two of the authors (ARS and JKM) and diagnoses for all cases were confirmed by routine hematoxylin and eosin staining. Both biopsy and resection specimens were incorporated but only one sample was used per patient. We only included cases that were classified as definitively invasive micropapillary carcinoma or typical urothelial carcinoma with retraction artifact. By definition, micropapillary tumors had small tight nests or balls of cells within retraction (lacunar) spaces and typically had foci with multiple tumor cell nests within a single space, back to back lacunar spaces, small branching micropapillae, and ring forms. Typical invasive urothelial carcinoma with retraction had larger confluent nests within spaces that typically were less prominent with only a small thin rim of space that conformed to the shape of the tumor nests. In our opinion, there are cases that are difficult to separate into micropapillary or nonmicropapillary by morphology. These ‘gray-zone’ cases would be diagnostically controversial and were not utilized in this study.
Immunohistochemical staining using antibodies against MUC1, CA125, and Her2Neu was performed on one 4-μm-thick formalin-fixed, paraffin-embedded representative section per case mounted on charged slides and baked at 60°C for 1 h. The primary antibodies used in the study are listed in Table 1. All cases were stained in parallel with appropriate positive and negative controls. Staining for MUC1 and CA125 was semiquantitatively scored as negative (0, <5% cells stained), focally positive (1+, 5–10% cells stained), positive (2+, 10–50% cells stained), or diffusely positive (3+, >50% cells stained), and a mean extent (ME, range 0–3) calculated. For the statistical evaluation, only the stroma-facing or ‘reverse-apical’ pattern of MUC1 reactivity was scored as positive. Staining intensity was semiquantitatively scored from 0 to 3+ and a mean intensity (MI, range 0–3) was calculated. Immunostaining for Her2Neu was scored based on the 2007 CAP/ASCO guidelines for invasive breast carcinoma.21 Statistical analysis used Fisher's two-tailed exact test, with significance set at P<0.05.
Results
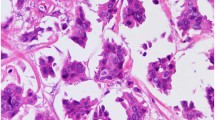
The age and stage distribution for both study and control cases is provided in Table 2. Of the 24 invasive micropapillary carcinomas evaluated, specimens included cystoprostatectomy (n=14), transurethral bladder tumor resection (n=7), cystectomy (n=2), and nephroureterectomy (n=1) and included 22 men and 2 women. Extent of micropapillary histology in the 24 invasive micropapillary carcinomas ranged from <10 to >50% (Figure 1a and b). Of the invasive urothelial carcinomas with retraction artifact evaluated, specimens included cystoprostatectomy (n=16), transurethral bladder tumor resection (n=4), nephroureterectomy (n=2), and cystectomy (n=2) and included 23 men and 1 woman. Among the 24 control invasive urothelial carcinomas with retraction artifact, the extent of retraction ranged from <10 to >50% (Figure 1c and d).
H&E morphology. (a) Neoplastic tumor nests of invasive micropapillary carcinoma free-floating in cleft-like spaces (original magnification × 100). (b) Multiple nests and papillae in single retraction spaces characteristic of invasive micropapillary carcinoma (original magnification × 400). (c) Typical invasive urothelial carcinoma frequently demonstrates retraction surrounding tumor nests (original magnification × 200). (d) Single tumor nests/aggregates within a single space and larger confluent nests are typical (original magnification × 400).
Immunohistochemical staining results for both invasive micropapillary carcinoma and invasive urothelial carcinoma with retraction artifact using MUC1, CA125, and Her2Neu immunostains are listed in Table 3.
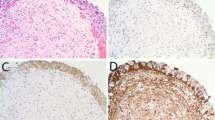
Of 24, 23 (96%) invasive micropapillary carcinomas showed positive MUC1 reactivity in a stroma-facing (basal) distribution with strong intensity and wide extent (Figure 2a). Among invasive urothelial carcinomas with retraction artifact, 15 of the 24 (63%) cases demonstrated basal MUC1 staining with strong intensity and wide extent. Intracytoplasmic and nonbasal membranous staining was also present in 9 of the 24 invasive micropapillary carcinoma cases, ranging from <10 to >50%, including 1 without any basal staining. Of the 24, 23 invasive urothelial carcinomas with retraction artifact cases also showed intracytoplasmic and nonbasal membranous reactivity ranging from <10 to >50% (Figure 2b), including 9 without a basal reactivity pattern. By our definition, cases without the basal MUC1 staining pattern were scored as negative for statistical analysis regardless of other cytoplasmic or membranous staining patterns.
Immunohistochemistry. (a) MUC1 in invasive micropapillary carcinoma demonstrates strong basal (stroma-facing, ‘reverse-apical’) reactivity. (b) MUC1 in typical invasive urothelial carcinoma with retraction also showed strong stroma-facing staining. (c) Although membranous reactivity for CA125 was more often seen in invasive micropapillary carcinoma, (d) typical invasive urothelial carcinoma with retraction also demonstrated staining with both strong intensity and extent. Uniform intense membrane staining of >30% of tumor cells (3+ score) with Her2Neu was seen in both (e) invasive micropapillary carcinoma and (f) typical invasive urothelial carcinoma with retraction. IMPC, invasive micropapillary carcinoma; IUC-R, invasive urothelial carcinoma with prominent retraction.
Membranous reactivity with CA125 was identified in 8 of the 24 (33%) invasive micropapillary carcinomas with both strong staining intensity and extent (Figure 2c). Among invasive urothelial carcinomas with retraction artifact, 3 of the 24 (13%) cases showed membranous staining, all with strong intensity and extent (Figure 2d).
Using 3+ membranous reactivity for Her2Neu as positive based on the 2007 CAP/ASCO guidelines for invasive breast carcinoma (defined as uniform intense membrane staining of >30% of tumor cells21), 6 of the 24 (25%) invasive micropapillary carcinoma and 2 of the 24 (8%) invasive urothelial carcinomas with retraction artifact showed Her2Neu-positive staining (Figure 2e and f). In addition, relaxing the threshold to include 2+ or 3+ membranous Her2Neu reactivity as positive, both invasive micropapillary carcinoma and invasive urothelial carcinoma with retraction artifact showed an equal number of positive cases (6 of 24, 25%).
The sensitivity and specificity for invasive micropapillary carcinoma vs invasive urothelial carcinoma with retraction artifact using MUC1, CA125, and Her2Neu are listed in Table 4. Antibody expression did not vary by stage distribution (data not shown). Previously reported immunohistochemical findings in invasive micropapillary carcinoma are summarized in Figure 3.
Discussion
The prognosis for invasive micropapillary carcinoma of the urinary tract is poor, and even in the absence of muscularis propria invasion in a biopsy, muscle invasion is often assumed.22 Because conservative therapy with intravesical bacillus Calmette-Guerin has shown poor success in invasive micropapillary carcinoma patients,13, 23, 24 some authors have proposed that early radical cystectomy be offered to patients with surgically resectable disease even in the absence of muscularis propria invasion on initial biopsy.23, 24 As such, the pathologic diagnosis of even a small focus of the micropapillary component may be therapeutically important.25
In this study, we compared the immunoprofile of a series of classic invasive micropapillary carcinomas to typical invasive urothelial carcinomas with retraction artifact using the three most promising markers (MUC1, CA125, and Her2Neu) derived from the micropapillary literature. Membranous CA125 reactivity in invasive micropapillary carcinoma was first reported by Johansson et al in 199913 in a study of 20 invasive micropapillary carcinomas with a sensitivity of 35% (n=20). Two additional studies have also reported CA125 expression in invasive micropapillary carcinoma, ranging from 100% (n=1)14 to 43% (n=7).19 Of these three studies, only Johansson et al13 compared CA125 expression in invasive micropapillary components (n=20) to components of typical invasive urothelial carcinoma within the same tumors (n=8) and reported a specificity of 100%. Similarly, Zhang et al in 200420 examined the expression of Her2Neu in invasive micropapillary carcinoma (n=10) vs conventional invasive urothelial carcinoma (n=59), reporting a sensitivity and specificity for micropapillary carcinoma of 100 and 57%, respectively. Regarding MUC1 antibody, a characteristic basal (‘reverse-apical’) pattern of reactivity in invasive micropapillary carcinoma was first reported by Nassar et al in 2004 (n=10).16 Compared to conventional invasive urothelial carcinoma (n=10), they reported 100% sensitivity and specificity for invasive micropapillary carcinoma. Basal MUC1 reactivity has also been reported in invasive micropapillary carcinoma (n=2) in two other case reports.8, 17
These previous studies of MUC1, CA125, and Her2Neu expression in invasive micropapillary carcinoma identification used typical invasive urothelial carcinoma as a control group,13, 16, 20 which is an infrequent problem in the morphologic differential of invasive micropapillary carcinoma. In our experience, when typical invasive urothelial carcinoma has an invasive pattern characterized by nests of neoplastic cells with a surrounding clear space closely resembling lymphovascular invasion26 (ie extensive retraction artifact), the distinction from invasive micropapillary carcinoma may become challenging. This difficulty is most pronounced when the invasive nests of neoplastic cells are relatively small. Given this potential for morphologic overlap in the face of emerging clinical relevance, we sought to determine the diagnostic utility of MUC1, CA125, and Her2Neu antibodies in the distinction of invasive micropapillary carcinoma from this specific group of typical urothelial carcinomas with extensive stromal retraction.
Invasive micropapillary carcinomas more commonly showed immunoreactivity for MUC1, CA125, and Her2Neu compared to typical invasive urothelial carcinoma with prominent retraction, but only MUC1 reached statistical significance. Although immunostaining for Her2Neu showed high specificity for invasive micropapillary carcinoma vs invasive urothelial carcinoma with retraction artifact (92%), it had poor sensitivity (25%) and did not reach statistical significance, even if 2+ or 3+ staining was interpreted as positive. Staining for CA125 also showed poor sensitivity (33%) despite a relatively high specificity (87%) for invasive micropapillary carcinoma compared to invasive urothelial carcinoma with retraction artifact. The lack of specificity (MUC1) and low sensitivity (CA125 and Her2Neu) of these evaluated markers limits their diagnostic utility in the distinction of invasive micropapillary carcinoma from the subgroup of typical urothelial carcinomas with extensive retraction artifact, the morphologically relevant control group.
The epidemiology of urothelial carcinoma variants is not well studied, so the incidence and/or prevalence of micropapillary carcinoma and typical invasive urothelial carcinoma with retraction are not known. Studies estimate that micropapillary carcinoma comprises approximately 0.7–2.2% of all urothelial tumors.1, 13 In our practice, approximately 5% of all invasive urothelial carcinomas have at least focal micropapillary features (unpublished data). Although we are not aware of any data specific to urothelial carcinomas with retraction artifact, based on our anecdotal experience we expect typical urothelial carcinoma with stromal retraction to be much more common. This would imply that based on relative frequency, immunohistochemical MUC1, CA125, and/or Her2Neu expression in a bladder tumor encountered in routine practice may more commonly represent a typical, nonmicropapillary carcinoma with stromal retraction rather than a true micropapillary carcinoma, underscoring the problem of using the immunophenotype for classification in this setting.
Although immunohistochemical expression of CA125 and Her2Neu in invasive micropapillary carcinoma has shown no definitive relationship to pathogenesis,13, 14, 19, 20 the more recently characterized MUC1 expression in micropapillary carcinomas of the urinary tract offers a plausible explanation for the neoplasm's aggressive behavior related to its histologic appearance.4, 16 The characteristic invasive micropapillary carcinoma morphology is depicted as being due to the ‘reverse polarity’ of tumors cells, whereby the stroma-facing (basal) cell surfaces acquire apical secretory properties.4, 16 Metalloproteinase secretion by invasive micropapillary carcinoma tumor cells, frequently present at the advancing edge of usual invasive urothelial carcinoma, is thought to facilitate neoplastic cell cluster detachment from stroma and ultimately tumor dissemination.4, 16 Ultrastructural examination identifying microvilli in stroma-facing cells27 as well as immunohistochemical studies highlighting the glycoprotein MUC1 in a reverse-polarity staining pattern4, 16 corroborates the ‘inside out’ micropapillary pattern pathogenesis. The MUC1 expression shared by both micropapillary carcinoma and the subset of nonmicropapillary urothelial carcinomas with stromal retraction artifact at least suggests the possibility of some biologic relationship between these two histologic types of bladder cancer. It is interesting to note that an association between extensive stromal retraction and adverse clinical features (ie nodal metastasis) is well described in invasive ductal carcinoma of the breast, even in the absence of micropapillary features.28, 29 These authors have proposed that both of these histologic types of breast carcinoma share an altered tumor–stromal interaction that may be involved in lymphatic spread.28, 29 Whether stromal retraction and/or MUC1 expression in typical (ie nonmicropapillary) urothelial carcinoma has any independent clinical significance has not been fully evaluated and warrants further investigation.
In summary, the present study shows that MUC1, CA125, and Her2Neu immunophenotypes do not have utility in the distinction of invasive micropapillary carcinoma from invasive urothelial carcinoma with retraction artifact. Given the potential implications for therapy, this distinction should continue to be based on morphology until more specific markers are identified.
References
Amin MB, Ro JY, el-Sharkawy T, et al. Micropapillary variant of transitional cell carcinoma of the urinary bladder. Histologic pattern resembling ovarian papillary serous carcinoma. Am J Surg Pathol 1994;18:1224–1232.
Amin MB, Tamboli P, Merchant SH, et al. Micropapillary component in lung adenocarcinoma: a distinctive histologic feature with possible prognostic significance. Am J Surg Pathol 2002;26:358–364.
Nagao T, Gaffey TA, Visscher DW, et al. Invasive micropapillary salivary duct carcinoma: a distinct histologic variant with biologic significance. Am J Surg Pathol 2004;28:319–326.
Nassar H . Carcinomas with micropapillary morphology: clinical significance and current concepts. Adv Anat Pathol 2004;11:297–303.
Sakamoto K, Watanabe M, De La Cruz C, et al. Primary invasive micropapillary carcinoma of the colon. Histopathology 2005;47:479–484.
Siriaunkgul S, Tavassoli FA . Invasive micropapillary carcinoma of the breast. Mod Pathol 1993;6:660–662.
Alvarado-Cabrero I, Sierra-Santiesteban FI, Mantilla-Morales A, et al. Micropapillary carcinoma of the urothelial tract. A clinicopathologic study of 38 cases. Ann Diagn Pathol 2005;9:1–5.
Munakata S, Tahara H, Kojima K, et al. Micropapillary urothelial carcinoma of the renal pelvis: report of a case and review of the literature. Med Sci Monit 2007;13:CS47–CS52.
Oh YL, Kim KR . Micropapillary variant of transitional cell carcinoma of the ureter. Pathol Int 2000;50:52–56.
Perez-Montiel D, Hes O, Michal M, et al. Micropapillary urothelial carcinoma of the upper urinary tract: clinicopathologic study of five cases. Am J Clin Pathol 2006;126:86–92.
Perez-Montiel D, Wakely PE, Hes O, et al. High-grade urothelial carcinoma of the renal pelvis: clinicopathologic study of 108 cases with emphasis on unusual morphologic variants. Mod Pathol 2006;19:494–503.
Vang R, Abrams J . A micropapillary variant of transitional cell carcinoma arising in the ureter. Arch Pathol Lab Med 2000;124:1347–1348.
Johansson SL, Borghede G, Holmang S . Micropapillary bladder carcinoma: a clinicopathological study of 20 cases. J Urol 1999;161:1798–1802.
Kuroda N, Tamura M, Ohara M, et al. Invasive micropapillary carcinoma of the urinary bladder: an immunohistochemical study of neoplastic and stromal cells. Int J Urol 2006;13:1015–1018.
Maranchie JK, Bouyounes BT, Zhang PL, et al. Clinical and pathological characteristics of micropapillary transitional cell carcinoma: a highly aggressive variant. J Urol 2000;163:748–751.
Nassar H, Pansare V, Zhang H, et al. Pathogenesis of invasive micropapillary carcinoma: role of MUC1 glycoprotein. Mod Pathol 2004;17:1045–1050.
Ohtsuki Y, Ochi K, Okada Y, et al. Micropapillary component of urothelial carcinoma detected in transurethral resection of bladder tumor (TUR-BT) tissues: a case report. Med Mol Morphol 2008;41:113–116.
Regalado JJ . Mixed micropapillary and trophoblastic carcinoma of bladder: report of a first case with new immunohistochemical evidence of urothelial origin. Hum Pathol 2004;35:382–384.
Samaratunga H, Khoo K . Micropapillary variant of urothelial carcinoma of the urinary bladder; a clinicopathological and immunohistochemical study. Histopathology 2004;45:55–64.
Zhang HSR, Ali-Fehmi R, Bianco F, et al. Characteristic immunohistochemical profiles and aggressive behavior of micropapillary and other histological variants and subtypes of urothelial carcinoma. Mod Pathol 2004;17 (Suppl 1):186A (782).
Wolff AC, Hammond ME, Schwartz JN, et al. American society of clinical oncology/college of American pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med 2007;131:18.
Zhai QJ, Black J, Ayala AG, et al. Histologic variants of infiltrating urothelial carcinoma. Arch Pathol Lab Med 2007;131:1244–1256.
Kamat AM, Dinney CP, Gee JR, et al. Micropapillary bladder cancer: a review of the University of Texas M. D. Anderson Cancer Center experience with 100 consecutive patients. Cancer 2007;110:62–67.
Kamat AM, Gee JR, Dinney CP, et al. The case for early cystectomy in the treatment of nonmuscle invasive micropapillary bladder carcinoma. J Urol 2006;175:881–885.
McKenney J . News in brief: the clinical management of ‘superficial’ (pT1/cT1) micropapillary carcinoma of the urinary bladder: are times changing? Adv Anat Pathol 2007;14:444–445.
McKenney JK, Gomez JA, Desai S, et al. Morphologic expressions of urothelial carcinoma in situ: a detailed evaluation of its histologic patterns with emphasis on carcinoma in situ with microinvasion. Am J Surg Pathol 2001;25:356–362.
Luna-More S, Gonzalez B, Acedo C, et al. Invasive micropapillary carcinoma of the breast. A new special type of invasive mammary carcinoma. Pathol Res Pract 1994;190:668–674.
Acs G, Dumoff KL, Solin LJ, et al. Extensive retraction artifact correlates with lymphatic invasion and nodal metastasis and predicts poor outcome in early stage breast carcinoma. Am J Surg Pathol 2007;31:129–140.
Acs G, Paragh G, Chuang ST, et al. The presence of micropapillary features and retraction artifact in core needle biopsy material predicts lymph node metastasis in breast carcinoma. Am J Surg Pathol 2009;33:202–210.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sangoi, A., Higgins, J., Rouse, R. et al. Immunohistochemical comparison of MUC1, CA125, and Her2Neu in invasive micropapillary carcinoma of the urinary tract and typical invasive urothelial carcinoma with retraction artifact. Mod Pathol 22, 660–667 (2009). https://doi.org/10.1038/modpathol.2009.16
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2009.16
Keywords
This article is cited by
-
Invasive Micropapillary Urothelial Carcinoma: an Uncommon and Underreported Variant in Cystectomy Specimens
Indian Journal of Surgical Oncology (2023)
-
Development and validation of a nomogram for urothelial cancer in patients with chronic kidney disease
Scientific Reports (2019)
-
High Grade T1 Papillary Urothelial Bladder Cancer Shows Prominent Peritumoral Retraction Clefting
Pathology & Oncology Research (2018)
-
Micropapillary morphology is an indicator of poor prognosis in patients with urothelial carcinoma treated with transurethral resection and radiochemotherapy
Virchows Archiv (2016)
-
Comparison of tyrosine kinase receptors HER2, EGFR, and VEGFR expression in micropapillary urothelial carcinoma with invasive urothelial carcinoma
Targeted Oncology (2015)