Abstract
The serum level of lactate dehydrogenase (LDH) is an important predictor of prognosis and treatment response in melanoma patients. It is unknown whether the expression of LDH-5 in tissue sections also has prognostic significance and whether it is related to the expression of the anti-apoptotic proteins, Bcl-2, Bcl-XL and Mcl-1, and endoplasmic reticulum stress protein glucose-regulated protein 78 (GRP78). Identification of an association between LDH-5 expression and anti-apoptotic proteins may have important therapeutic implications for melanoma patients. Sections from 159 pigmented lesions, including nevi and melanoma at different stages of progression were studied by immunohistochemistry. Correlation of LDH-5 expression with clinicopathological factors and with the expression of Bcl-2, Bcl-XL, Mcl-1 and GRP78 was examined. LDH-5 was detected at low levels in 6 of 10 compound nevi (60%) and 6 of 10 dysplastic nevi (60%). The percentage of positive cases was greater in thin (≤1.0 mm) (74%) and thick primary melanoma (>1.0 mm) (95%) and in metastatic melanoma in the skin (100%) and lymph node (81%). The immunoreactive score was highly related to progression of melanoma (P<0.0001). LDH-5 expression was positively associated with increasing tumor thickness (P=0.02) and dermal tumor mitotic rate (P=0.02). LDH-5 above the median immunoreactive score was associated with reduced disease-free survival and overall survival (P<0.02). LDH-5 expression was negatively associated with Bcl-2 expression. In contrast, LDH-5 expression was strongly associated with Bcl-XL and Mcl-1 expression and also positively associated with GRP78 expression (P<0.0001). The low Bcl-2 expression in melanomas with high LDH-5 expression provides an explanation for the poor response of patients with high serum LDH levels to treatment with the Bcl-2 antisense drug ‘Genasense’. The strong correlation of LDH-5 expression with Mcl-1 expression suggests that treatment strategies inhibiting the activity of Mcl-1 in melanoma patients should be investigated.
Similar content being viewed by others
Main
Previous studies have shown that elevated levels of serum lactate dehydrogenase (LDH) in melanoma patients are associated with adverse prognosis.1, 2, 3, 4 Serum LDH level is known to be the most specific marker of progressive disease in American Joint Committee on Cancer Stage IV melanoma patients3, 5 and is increasingly used by sponsors of clinical trials to exclude patients with adverse prognosis. However, the expression of LDH in human melanoma tissues has not, to the best of our knowledge, been previously assessed.
Lactate dehydrogenase is encoded by two genes, LDH-A (the M subunit-muscle type) and LDH-B (the H subunit-heart type), which give rise to two polypeptide chains that form five isoenzymes, depending on the combinations of the subunits comprising the chains. LDH-5 is composed of four M subunits.6 The major role of LDH in normal cellular homeostasis is to enhance the catalysis of pyruvate to lactate to produce adenosine triphosphate (ATP). LDH-5 is the most efficient isotype in this catalysis.7 This process is less efficient for ATP production than oxidative phosphorylation in the Krebs citric acid cycle (which predominates in aerobic glucose metabolism). However, the catalysis of pyruvate is the major mechanism of ATP production in most melanomas because of changes in glucose metabolism referred to as the Warburg effect.8, 9 Another isotype LDH-1 catalyses the aerobic oxidation of pyruvate by pyruvate dehydrogenase. A previous study showed that LDH-1 was expressed in all living cells, including normal and malignant cells.7 The mechanism of action of the other three isotypes of LDH is unclear at present.
Increased levels of LDH within cells occur largely because of upregulation by the transcription factor ‘hypoxia-inducible factor 1α’, which in turn is upregulated by hypoxia10 and by the endoplasmic reticulum stress response resulting from hypoxia, hypoglycemia and other demands of the malignant proliferation.11, 12 The stronger the drive of the malignant process, the more likely the cell is to undergo endoplasmic reticulum stress. Endoplasmic reticulum stress activates three well-defined pathways referred to as the unfolded protein response. The pathways are those activated by the proteins, ATF6 and PERK, and the inositol-requiring enzyme, IRE 1. As reviewed elsewhere,11 induction of endoplasmic reticulum stress in melanoma results in several compensatory mechanisms which include upregulation of the glucose-regulated protein 78 (GRP78) and the Bcl-2 family member protein Mcl-1.13 Previous immunohistological studies showed decreased levels of Bcl-2 with progression of melanoma.14
In this study, we assessed tissue expression of LDH-5 in benign melanocytic tumors and in melanomas at different stages of progression, in order to determine whether LDH-5 expression in tissue sections is an independent predictor of prognosis. In view of the association of LDH-5 with endoplasmic reticulum stress and changes in Bcl-2 family anti-apoptotic proteins and GRP78, we also examined whether LDH-5 expression in melanocytic tumors was related to the expression of these proteins. This is particularly relevant in view of the poor response of patients with high serum LDH treated with antisense to Bcl-2 in the so-called Genta trial.15
Materials and methods
Cases
Archival paraffin tissue blocks of 159 melanocytic lesions excised between 2000 and 2003 were retrieved from the Department of Tissue Pathology and Diagnostic Oncology at Royal Prince Alfred Hospital, Sydney, Australia. There were (i) 10 compound nevi, (ii) 10 dysplastic nevi, (iii) 9 nevi associated with melanoma, (iv) 98 melanomas, including 12 in situ melanomas, 34 thin primary cutaneous melanomas (≤1.0 mm in thickness), 52 thick primary cutaneous melanoma (>1.0 mm in thickness), (v) 16 subcutaneous (SC) melanoma metastases, including one metastasis in skeletal muscle, and (6) 16 lymph node metastases. All of the melanoma patients were treated at the Melanoma Institute Australia/Sydney Melanoma Unit between January 2000 and March 2002. Hematoxylin and eosin (H & E)-stained sections of all 159 cases were reviewed. ‘Nevi associated with melanoma’ were defined as nevi that were intimately associated with melanoma. Although some of the associated nevi may have been precursor lesions, it was not possible to determine this histopathologically. The primary invasive melanomas comprised 48 cases of superficial-spreading melanoma, 28 cases of nodular melanoma, 4 cases of desmoplastic melanoma, 2 cases of acral lentiginous melanoma, 3 cases of lentigo maligna melanoma (melanoma arising in a Hutchinson's melanotic freckle) and 1 unclassifiable melanoma. All patients with primary melanoma had regular follow-up at the Melanoma Institute Australia/Sydney Melanoma Unit (median follow-up period 62.5 months).
Immunohistochemistry
Sections of 5 μm thickness were cut from the formalin-fixed, paraffin-embedded tissue block from each tumor. Sections were deparaffinized in xylene and rehydrated through decreasing concentrations of alcohol. Antigen retrieval was carried out as reported previously14, 16 in EDTA buffer pH 8.6 by heating in a microwave oven for 5 min and repeating this three times. Rabbit anti-human LDH-5 antibody (polyclonal IgG, Abcam, Cambridge, UK, Cat ab53010) was added at a dilution of 1:150 in Tris buffer for 1 h. Mouse anti-human Bcl-XL (H-5) antibody (monoclonal IgG1, Santa Cruz, San Diego, CA, USA, Cat SC-8392) was added at 1:400 dilution in Tris buffer, and rabbit anti-human Mcl-1 antibody (polyclonal IgG, DAKO, Carpenteria, CA, USA, Lot 057) at a dilution of 1:30 in Tris buffer was incubated on the sections at room temperature for 1 h. Rabbit anti-human GRP 78 antibody (polyclonal IgG, Santa Cruz, Cat SC-13968, clone H-129) was added at a dilution of 1:100 in Tris buffer for 1 h. The Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA) was used to bind the antibodies according to the manufacturer's instructions and the binding sites were visualized using the DAB kit (DAKO, Cat K3466). Anti-human Bcl-2 mouse antibody (monoclonal IgG, DAKO, Cat M0887, clone 124) was added at a dilution of 1:50 in Tris buffer for 1 h. Rabbit anti-mouse immunoblubin (DAKO) was used to detect the primary antibodies and was visualized using ‘ENVISION’-PLUS-HRP (Rabbit, DAKO, Cat K4003) and DAB kit (DAKO). The sections were counterstained with Harris hematoxylin. Negative controls were performed by omission of the primary antibody in each experiment. Colonic carcinoma, normal lymph node and normal breast were used as positive controls as appropriate.
The percentage of positive cells (0–100%) was estimated based on the evaluation of the immunohistochemically stained sections by a pathologist experienced in the interpretation of immunohistochemistry. Intensity of staining (intensity score) was judged on an arbitrary scale of 0–4+: no staining (0), weakly positive staining (1+), moderately positive staining (2+), strongly positive staining (3+) and very strongly positive staining (4+). An immunoreactive score was derived by multiplying the percentage of positive cells with staining intensity divided by 10. The distribution of staining was classified as focal or diffuse. Localization of staining (nuclear, membranous or cytoplasmic) was also recorded.
Statistical Analysis
Statistical analysis was carried out using Microsoft Excel 2000 software and ‘JMP Statistics Made VisualTM’ software (SAS Institute, Turnbull, CT, USA). The one-way ANOVA two-tailed t-test was used to assess differences in the expression of LDH-5 between different types of melanocytic tumors. Multiple comparisons for all pairs were carried out using the Tukey-Kramer HSD method and Student's t-test was also used for all pairs. The comparisons of the LDH-5 expression in histopathological subtypes of primary melanoma, excluding acral lentiginous melanoma, lentigo maligna melanoma and unclassified melanoma, were carried out using the one-way ANOVA two-tailed t-test and the Tukey-Kramer HSD method. The correlations between LDH-5 expression in primary melanoma and Breslow thickness and dermal mitotic rate were assessed using regression analysis. Correlations between LDH-5 expression and Bcl-2, Bcl-XL, Mcl-1 and GRP 78 expression in melanocytic lesions were also examined by regression analysis.
Disease-free survival (DFS) and overall survival (OS) were calculated using Kaplan–Meier univariate estimates. Differences in DFS and OS between patients with melanoma according to LDH-5 expression (above and below the median immunoreactive score) were compared using log-rank and χ2 statistical methods, respectively. The univariate analysis was followed by multivariate analysis according to the Cox Proportional Hazards Model using SPSS 16.0 software (SPSS, Chicago, IL, USA). A P-value of <0.05 was considered statistically significant.
This study received ethics approval from the Sydney South West Area Health Service NSW Health Ethics Review Committee of Royal Prince Alfred Hospital (Protocol No X04-0061).
Results
Clinicopathological Features
The melanocytic lesions studied and the corresponding patient demographics are summarized in Table 1. The age of the patients ranged from 16 to 93 years with a median of 58 years. Fifty patients were female and 109 were male. The age of patients with primary melanoma ranged from 23 to 89 years with a median age of 62 years. Twenty-nine (30%) of the primary melanoma group of patients were female and 69 (70%) were male. The Breslow thickness of the primary melanoma ranged from 0 mm (in situ melanoma) to 16 mm. Forty-six of the melanomas were ≤1 mm in thickness, including in situ melanoma, and 52 were >1 mm in thickness. The majority of the melanomas were of the superficial spreading (48 cases) or the nodular (28 cases) histological subtypes.
LDH-5 Expression
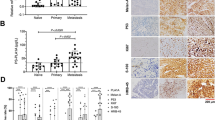
As shown in Table 2, LDH-5 was detected in 6 of 10 compound nevi (60%), 6 of 10 dysplastic nevi (60%), 8 of 9 nevi associated with melanoma (89%) and 8 of 12 in situ melanomas (67%). The percentage of positive cases, however, was greater in thin melanoma (74%), thick melanoma (95%), subcutaneous metastases (100%) and lymph node metastases (81%). The mean percentages of cells positive for LDH-5 in compound nevi, dysplastic nevi, nevi associated with melanoma, in situ melanoma, thin melanoma, thick melanoma, subcutaneous melanoma metastases and lymph node metastases were 28, 34, 49, 55.8, 65.9, 77.3, 93.8 and 64.4%, respectively. LDH-5 staining was located in the cytoplasm of the cells. The intensity of staining varied from 0 to 4+. Staining was stronger in thick primary melanomas and metastases. In contrast, expression was much weaker in compound nevi, dysplastic nevi, nevi associated with melanoma and in situ melanoma (Figure 1a–f). The immunoreactive scores were higher in thin melanoma (mean immunoreactive score=11), thick melanomas (mean immunoreactive score=19.6), subcutaneous melanoma metastases (mean immunoreactive score=25.1) and lymph node metastases (mean immunoreactive score=17.9), compared with those in compound nevi (mean immunoreactive score=2), dysplastic nevi (mean immunoreactive score=3.9), nevi associated with melanoma (mean immunoreactive score=7.8) and in situ melanoma (mean immunoreactive score=6.4) (Figure 2a). These differences were significant using the one-way ANOVA two-tailed t-test (P<0.0001).
LDH-5 expression in different types of melanocytic tumors. (a) Negative staining in a compound nevus; IRS=0; ( × 200). (b) Weak and superficial staining in a dysplastic nevus with 10% of cells positive; IRS=1; ( × 200). (c(i)) A melanoma in situ with 100% of cells positive; IRS=10; ( × 200). (c(ii)) Negative staining in a melanoma in situ; IRS=0; ( × 200). (d) A melanoma ≤1.0-mm thick with 100% of cells positive; IRS=20; ( × 200). (e) A melanoma >1.0-mm thick with 100% of cells positive; IRS=30; ( × 400). (f(i)) A subcutaneous melanoma metastasis with 100% of cells positive; IRS=40; ( × 200). (f(ii)) A lymph node metastasis with 100% of cells positive; IRS=20; ( × 200).
(a) Mean immunoreactive score of cells positive for LDH-5 in different types of melanocytic tumors. N, nevus; M, melanoma; Met, metastatic; LN, lymph node; n, number; thick and thin, Breslow thickness >1.0 mm and ≤1.0 mm (not including in situ melanoma), respectively; Y bar indicates one standard error. Statistical differences by Student's t-test and Tukey-Kramer tests were as follows: *Thin M vs LN met, DN P<0.05 and vs CN P<0.005 (Student's t-test); Thin M vs thick M P<0.001, vs SC met P<0.0001 (Student's t-test and Tukey-Kramer test). **Thick M vs CN P<0.0001, vs DN and in situ M P<0.0005, vs Thin M P<0.001 and vs N+M P<0.005 (Student's t-test and Tukey-Kramer test). ***SC met vs CN, DN, in situ M, Thin M P<0.0001 and vs N+M P<0.001 (Student's t-test and Tukey-Kramer test). ****LN met vs CN, DN P<0.005, vs in situ M and thin M P<0.05. (b) Mean immunoreactive score of cells positive for LDH-5 in different histopathological types of primary melanoma. n=number; Y bar indicates one standard error. Statistical differences by Student's t-test and Tukey-Kramer tests were as follows: *in situ M vs nodular M P=0.0002, vs superficial-spreading M P=0.0117.
The correlation of LDH-5 expression with histopathological subtypes of primary melanoma is shown in Figure 2b. The LDH-5 immunoreactive score mean was significantly lower in in situ melanoma (immunoreactive score=6.4) compared with that seen in superficial-spreading melanoma (mean immunoreactive score=15) (P<0.0005) and nodular melanoma (mean immunoreactive score=19.7) (P<0.05). However, there was no significant difference in immunoreactive score between superficial-spreading melanoma and nodular melanoma.
Relationship of LDH-5 Expression to Known Prognostic Features in Primary Melanoma
As shown in Figure 3a and b, LDH-5 expression was correlated with Breslow thickness and dermal mitotic rate. The immunoreactive score of LDH-5-positive cells increased with increasing tumor thickness (P=0.02) and with increasing dermal tumor mitotic rate (P=0.02).
(a) Immunoreactive score of LDH-5-positive tumor cells in primary melanoma correlated with Breslow thickness. Increasing LDH-5 expression was associated with increased tumor thickness (R2=0.54, P=0.02). (b) Immunoreactive score of LDH-5-positive tumor cells in primary melanoma correlated with dermal tumor mitotic rate. LDH-5 expression was increased with increasing dermal tumor mitotic rate (R2=0.5984, P=0.015).
Expression of Bcl-2 Family Proteins (Bcl-2, Bcl-XL, Mcl-1) and Endoplasmic Reticulum Stress Protein (GRP78)
Immunostaining for Bcl-2 family proteins including, Bcl-2, Bcl-XL and Mcl-1, and endoplasmic reticulum stress protein GRP78 was carried out on 135 cases. Bcl-2 was expressed in all benign nevi and thin melanomas but expression was reduced in thick melanomas, subcutaneous metastases and lymph node metastases. In contrast, Bcl-XL and Mcl-1 were expressed at lower levels in nevi and thin melanomas compared with Bcl-2, but their expression was much higher in thick melanomas, in subcutaneous metastases and in lymph node metastases. GRP78 expression was low in benign nevi and in situ melanomas but increased progressively in thin melanomas, thick melanomas, subcutaneous metastases and lymph node metastases.
Relationship of LDH-5 Expression to the Expression of Bcl-2 Family Proteins and GRP78
The graphs in Figure 4a–d show that LDH-5 expression tended to be negatively associated with Bcl-2 expression (P=0.3). In contrast, LDH-5 expression was strongly associated with Bcl-XL and Mcl-1 expression (P<0.0001). LDH-5 expression was also positively associated with GRP78 expression (P<0.0001).
Relationship of LDH-5 Expression to Patient Survival
Follow-up data were available in 93 of 98 melanoma patients (median follow-up 62.5 months); 21 patients (23%) developed recurrent melanoma (defined as including locoregional recurrences and distant metastases). Among those, 20 patients (22%) died of melanoma. All recurrences occurred in patients with thick melanoma. In all, 93 patients were grouped above and below the median values of IRS (median=15) for LDH-5. Comparison of DFS and OS in patients with an LDH-5 immunoreactive score above and below median value showed that DFS (χ2=5.661, P=0.017) and OS (χ2=5.084, P=0.024) were lower in the patients with LDH-5 immunoreactive score >15 (n=39) than in the patients with LDH-5 immunoreactive score ≤15 (n=54) (Figure 5a and b). Multivariate analysis showed that expression of LDH-5 was not an independent predictor of DFS or OS, but was dependent largely on tumor thickness, Clark level, ulceration and vascular and neural invasion (Tables 3A and B).
Kaplan–Meier estimates of disease free survival (DFS) and overall survival (OS) after excision of primary cutaneous melanoma. (a) DFS of patients with LDH-5-positive cells IRS ≥15 (median score) vs patients with LDH-5-positive cells IRS <15. (b) OS of patients with LDH-5-positive cells IRS ≥15 vs patients with LDH-5-positive cells IRS <15.
Discussion
This study is the first to examine in detail the expression of LDH-5 in human melanoma using immunohistochemistry on paraffin sections of excised tumors. As discussed in detail below, the identification of a negative association between LDH-5 expression and Bcl-2 expression and a strong association between LDH-5 expression and Bcl-XL and Mcl-1 expression may have important therapeutic implications for melanoma patients.
The results show that LDH-5 expression is closely related to progression of melanocytic tumors, being barely detectable in nevi but strongly expressed in thick primary melanoma and in metastatic melanoma. Although LDH-5 expression predicted both DFS and OS on univariate analysis, it was not an independent marker of prognosis in primary melanoma on multivariate analysis. Instead, LDH-5 expression was related to well-established prognostic markers, such as thickness, Clark level, ulceration and mitotic rate, and confirms that LDH is another marker of the degree of malignancy in melanoma.17 Given that LDH-5 expression was not an independent marker of prognosis, there would appear to be little benefit in including LDH expression in the routine pathological assessment of primary cutaneous melanoma. Whether there would be merit in assessment of prognosis in metastatic melanoma is not clear from this study and requires further investigation. For example, it may be useful to assess whether LDH-5 expression in metastatic melanoma in sentinel lymph nodes correlates with the spread of tumor to non-sentinel lymph node sites.
Previous studies have shown that LDH-5 expression in tissue sections of lung and colorectal adenocarcinomas was associated with aggressive tumor phenotypes, and furthermore, was a more sensitive indicator of prognosis than serum LDH levels.7, 18 Whether the latter also applies in melanoma is not known as serum LDH levels were not available for the patients in this study.
Of potential therapeutic importance was the finding that LDH-5 expression was strongly correlated with the expression of the anti-apoptotic proteins Bcl-XL and Mcl-1, but not Bcl-2. There was a trend toward lower expression of Bcl-2 in sections with high LDH-5 expression. This may explain the poor response of patients with high serum LDH levels to treatment with the Genasense antisense drug against Bcl-2,15 as the expression of Bcl-2 in the unresponsive tumors may have been too low for the treatment to have any effect. The finding of a strong correlation with Mcl-1 expression is of much interest, as our in vitro studies have shown that Mcl-1 is the main anti-apoptotic protein that protects melanoma from endoplasmic reticulum stress-induced apoptosis.13 The findings in the current study therefore provide further strong support for the investigation of treatment strategies that inhibit the anti-apoptotic activity of Mcl-1. The broad-spectrum anti-apoptotic drug, Obatoclax, is one such drug but several other strategies warrant investigation.19
LDH-5 was positively associated with the endoplasmic reticulum stress protein GRP78. This finding is consistent with upregulation of both proteins as part of the unfolded protein response during endoplasmic reticulum stress.20 GRP78 is upregulated by all three of the unfolded protein response pathways and was shown to be involved in resistance of melanoma cells to chemotherapeutic agents. Previous immunohistological studies have shown that its expression is related to progression in melanoma.21 The gene coding for Bcl-2 was shown to downregulated by the transcription factor, Gadd 153, which is activated by endoplasmic reticulum stress.22 Mcl-1 levels also increase during endoplasmic reticulum stress, although the mechanism by which this occurs is not clear.22 The results of this study therefore show that LDH-5 expression in melanoma identifies a more malignant phenotype that is associated with upregulation of proteins protecting against death of melanoma cells. The findings provide support for new therapeutic strategies aimed at downregulation of anti-apoptotic proteins, such as GRP78 and Mcl-1.
References
Sirott MN, Bajorin DF, Wong GY, et al. Prognostic factors in patients with metastatic malignant melanoma. A multivariate analysis. Cancer 1993;72:3091–3098.
Eton O, Legha SS, Moon TE, et al. Prognostic factors for survival of patients treated systemically for disseminated melanoma. J Clin Oncol 1998;16:1103–1111.
Deichmann M, Benner A, Bock M, et al. S100-beta.melanoma-inhibiting activity, and lactate dehydrogenase discriminate progressive from nonprogressive American Joint Committee on Cancer Stage IV melanoma. J Clin Oncol 1999;17:1892–1896.
Meral R, Duranyildiz D, Tas F, et al. Prognostic significance of melanoma inhibiting activity levels in malignant melanoma. Melanoma Res 2001;11:627–632.
Balch CM, Buzaid AC, Soong S-J, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001;19:3635–3648.
Giatromanolaki A, Sivridis E, Gatter KC, et al. Lactate dehydrogenase 5 (LDH-5) expression in endometrial cancer relates to the activated VEGF/VEGFR2(KDR) pathway and prognosis. Gynecol Oncol 2006;103:912–918.
Koukourakis M, Giatromanolaki A, Sivridis E . Lactate dehydrogenase isoenzymes 1 and 5: differential expression by neoplastic and stromal cells in non-small cell lung cancer and other epithelial malignant tumors. Tumor Biol 2003;24:199–202.
Pan JG, Mak TW . Metabolic targeting as an anticancer strategy: dawn of a new era? Sci STKE 2007;381:pe14.
Warburg O . On the origin of cancer cells. Science 1956;123:309–314.
Brahimi-Horn MC, Pouyssegur J . Harnessing the hypoxia-inducible factor in cancer and ischemic disease. Biochem Pharmacol 2007;73:450–457.
Hersey P, Zhang XD . Adaptation to ER stress as a driver of malignancy and resistance to therapy in human melanoma. Pigment Cell Melanoma Res 2008;21:358–367.
Werno C, Zhou J, Brune B . A23187, ionomycin and thaosigargin upregulate mRNA of HIF-1alpha via endoplasmic reticulum stress rather than a rise in intracellular calcium. J Cell Physiol 2008;215:708–714.
Jiang CC, Lucas K, Avery-Kiejda KA, et al. Up-regulation of Mcl-1 is critical for survival of human melanoma cells upon ER stress. Cancer Res 2008;68:6708–6717.
Zhuang L, Lee CS, Scolyer RA, et al. Mcl-1, Bcl-XL and Stat3 expression are associated with progression of melanoma whereas Bcl-2, AP-2 and MITF levels decrease during progression of melanoma. Mod Pathol 2007;20:416–426.
Bedikian AY, Millward M, Pehamberger H, et al. Bcl-2 antisense (oblimersem sodium) plus dacarbazine in patients with advanced melanoma: The Oblimersen Melanoma Study Group. J Clin Oncol 2006;24:4738–4745.
Shi S-R, Shi Y, Taylor CR . Immunohistochemistry and Molecular Morphology. Eaton Publishing: USA, 2000;311–320.
Walenta S, Mueller-Kliese WF . Lactate: mirror and motor of tumor malignancy. Semin Radiat Oncol 2004;14:267–274.
Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Lactate dehydrogenase 5 expression in operable colorectal cancer: strong association with survival and activated vascular endothelial growth factor pathway—a report of the tumor angiogenesis research group. J Clin Oncol 2006;24:4301–4308.
Schimmer AD, O’Brien S, Kantarjian H, et al. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin Cancer Res 2008;14:8295–8301.
Jiang CC, Mao ZG, Avery-Kiejda KA, et al. Glucose-regulated protein 78 antagonizes cisplatin and adriamycin in human melanoma cells. Carcinogenesis 2009;30:197–204.
Zhuang L, Scolyer RA, Lee CS, et al. Expression of glucose-regulated stress protein GRP78 is related to progression of melanoma. Histopathology 2009;54:462–470.
Kim B, Emi M, Tanabe K, et al. Role of the unfolded protein response in cell death. Apoptosis 2006;11:5–13.
Acknowledgements
We thank the staff of the Department of Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital, especially Dianne Maguire and Trina Lum, for their assistance with immunohistochemistry. We also thank the staff of the Oncology and Immunology Unit of the Calvary Mater Newcastle for their assistance and helpful comments, and Susan Wood from the Melanoma Institute Australia/Sydney Melanoma Unit Database for follow-up information on the patients in the study. This study was supported by funds from the NHMRC program grant on melanoma. Support from the Cancer Institute New South Wales is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhuang, L., Scolyer, R., Murali, R. et al. Lactate dehydrogenase 5 expression in melanoma increases with disease progression and is associated with expression of Bcl-XL and Mcl-1, but not Bcl-2 proteins. Mod Pathol 23, 45–53 (2010). https://doi.org/10.1038/modpathol.2009.129
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2009.129
Keywords
This article is cited by
-
LDHA induces EMT gene transcription and regulates autophagy to promote the metastasis and tumorigenesis of papillary thyroid carcinoma
Cell Death & Disease (2021)
-
Prognostic role of glycolysis for cancer outcome: evidence from 86 studies
Journal of Cancer Research and Clinical Oncology (2019)
-
Equating salivary lactate dehydrogenase (LDH) with LDH-5 expression in patients with oral squamous cell carcinoma: An insight into metabolic reprogramming of cancer cell as a predictor of aggressive phenotype
Tumor Biology (2016)
-
Oncogenic Activation of MEK/ERK Primes Melanoma Cells for Adaptation to Endoplasmic Reticulum Stress
Journal of Investigative Dermatology (2014)
-
Prognostic Impact of PHIP Copy Number in Melanoma: Linkage to Ulceration
Journal of Investigative Dermatology (2014)