Abstract
House dust mites (HDMs) induce allergic asthma in sensitized individuals, although how HDMs activate immature mucosal dendritic cells (DCs) to render the T helper cell type 2 (Th2)-mediated immune response is unclear. In this study, our results showed a significant calcium-dependent lectin binding of Dermatophagoides pteronyssinus (Der p) extracts to DC-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN), the C-type lectin receptors (CLRs) of DCs. Moreover, monocyte-derived DCs (MDDCs) of Der p-sensitized asthmatics (AS) exhibited decreased expression of DC-SIGN, increased endocytosis, and impaired differentiation of DC precursors. The Der p-induced downregulation of DC-SIGN expression in the differentiation of immature MDDCs may be because of the internalization of Der p-DC-SIGN complex. MDDCs of AS produced more interleukin (IL)-6 and less IL-12p70 cytokines when stimulated with lipopolysaccharide (LPS) or Der p than those of nonallergic controls (NC). In the co-culture experiments, MDDCs pretreated with Der p induced GATA-3 expression and IL-4 cytokine productions in naive CD4+ T cells. These effects of Der p on the differentiation and function of MDDCs could be partially blocked by anti-DC-SIGN antibodies. In conclusion, our results suggest a critical step of allergen sensitization that involves CLRs in the innate immune response of DCs, which may provide a therapeutic or preventive potential for allergic asthma.
Similar content being viewed by others
Introduction
Dendritic cells (DCs) are highly specialized antigen-presenting cells that regulate innate and adaptive immune responses and, as a result, play an important role in the pathogenesis of asthma and allergic rhinitis.1, 2, 3 DCs have the capacity to distinguish among different pathogenic compounds through the expression of various pattern-recognition receptors that recognize specific pathogen-associated molecular patterns. Examples are the families of the Toll-like receptors (TLRs) and the C-type lectin-like receptors (CLRs),4 including dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN) and macrophage mannose receptor, that recognize carbohydrate structures on pathogens and self-glycoproteins.5 In fact, the final adaptive T-cell immune response may be determined by the differentiation and maturation status, expression profile of co-stimulatory molecules, and specific pattern-recognition receptors used in the recognition of antigens by DCs.6, 7
Although the mechanisms by which allergens activate DCs and induce pathogenesis of T helper cell type 2 (Th2)-mediated allergy diseases remain to be clarified, separate studies have shown that molecules derived from pollen grains, including phytoprostanes, can condition purified human DCs into Th2-polarizing antigen-presenting cells.8, 9 Similarly, allergens derived from the house dust mite (HDM), Dermatophagoides pteronyssinus (Der p), induce differential changes in DCs from individuals with HDM allergy, but not healthy individuals,10, 11 and these allergen-conditioned DCs may preferentially induce a Th2-polarized immune response.12
Previously, we have demonstrated that extracts from dust mites (Dermatophagoides farinae) can directly activate innate immune cells such as alveolar macrophages13 and mast cells14 and can induce Th2 cytokine response without previous in vitro or in vivo sensitization. Recent studies have suggested that allergen-induced DC activation and inflammation may involve TLRs and/or CLRs on the antigen-presenting cells.15, 16, 17, 18 Hammad et al.15 found that HDMs activated TLR4 on the pulmonary epithelium and elicited allergen-driven Th2 responses via the generation of interleukin (IL)-25, IL-33, and thymic stromal lymphopoietin. We also found that HDM-induced nitric oxide and tumor necrosis factor-α (TNF-α) production of activated alveolar macrophages occurred via stimulation of CD14/TLR4 surface receptors.16 Mite allergens were also reported to bind to the CLR Dectin-2 on DCs that activate arachidonic acid metabolism and activate innate immune cells to promote allergic inflammation.17 Moreover, HDM was reported to decrease the expression of DC-SIGN on the surface of DCs via activation of its inherent protease activity.18 Nonetheless, the function of DC-SIGN in allergen-induced DC activation and its role in the polarization of allergen-specific Th2 immune response remains unclear.
To answer these questions, we have cultured monocyte-derived dendritic cells (MDDCs) from peripheral blood mononuclear cells of Der p-sensitive allergic asthmatics (AS) and nonallergic controls (NC) to evaluate the role of DC-SIGN in the polarization of Th2 cytokine response in response to Der p. Our data indicate that immature MDDCs internalized Der p allergen through DC-SIGN and, after maturation, promote Th2 polarization on naive CD4+ T cells. These results suggest a critical step of allergen sensitization that involved DC-SIGN in the innate immune response of human DCs.
Results
Effect of Der p allergen on the MDDC differentiation
The immunophenotypes of our cultured MDDCs reflected CD1a−CD14+ monocytes differentiated into CD1a+CD14− immature DCs. These MDDCs were confirmed to be negative for the cell surface receptor CD14 and positive for the receptors HLA-DR, CD11c, CD80, CD83, and CD86 (data not shown). To address the effect of Der p on MDDC differentiation, monocytes were cultured as described in the absence (medium-MDDCs) or presence of increasing concentrations of Der p allergen (Der p-MDDCs). Incubation of MDDCs with 10 μg ml–1 of Der p resulted in a significant reduction of CD1a+CD14− cells compared with controls (56.93±4.29 vs. 76.72±3.08%, respectively, P<0.05; Figure 1a and b). The suppressive effect of Der p allergen on differentiation of DCs from monocytes was observed in both Der p-sensitive AS and Der p-nonsensitive NC (Figure 1c). However, there were no significant differences in the percentages of CD1a+CD14− cells between AS and NC regardless of whether or not culture conditions included Der p (Figure 1c).
The effect of Dermatophagoides pteronyssinus (Der p) on monocyte-derived dendritic cell (MDDC) differentiation. (a) Representative experiment of flow cytometry for the cell surface expression of CD14 and CD1a in MDDCs incubated with Der p (Der p-MDDCs) or medium alone (medium-MDDCs). (b) The percentages of CD14−CD1a+ cells in medium-MDDCs and Der p-MDDCs were quantitated by flow cytometry. (c) The percentages of CD14−CD1a+ in medium-MDDCs and Der p-MDDCs were quantitated and compared between nonallergic controls (NC; n=12) and allergic asthmatics (AS; n=17). Data were analyzed using Student's t-test. *P<0.05; **P<0.01.
The interaction between Der p extracts and DC-SIGN
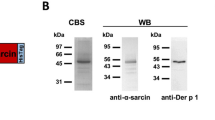
To know whether there is different expression levels of CLRs in DCs between AS and NC, MDDCs were cultured from peripheral blood mononuclear cells of our study subjects in the condition that was described in Methods. There were no significant differences in expression levels of the DC maturation markers such as HLA-DR, CD80, CD86, and macrophage mannose receptor in the MDDCs collected from NC and AS (Supplementary Figure S1 online). In contrast, MDDCs from AS displayed significantly lower levels of DC-SIGN when compared with NC (41.53±2.91 vs. 55.35±4.96, P<0.05, Figure 2a). Moreover, the mean fluorescence intensity (MFI) and percentages of MDDCs expressing DC-SIGN were markedly decreased with Der p treatment when compared with medium alone in AS (28.0±1.5 vs. 41.5±2.9; 55.6±7.4 vs. 77.3±7.3%; P<0.05) and NC (28.5±2.6 vs. 55.4±4.9; 52.9±9.9 vs. 85.6±7.4%; P<0.05; Figure 2b). To test whether C-type lectin receptor, DC-SIGN, can bind to mite allergen, Figure 2c shows recombinant DC-SIGN (1 μg ml–1) bound to various concentrations of solid-phase coated Der p extracts in a dose-dependent manner, but not to bovine serum albumin (data not shown). The removal of calcium by EDTA (10 mM) as well as adding maltose (50 mM) in the buffer inhibited the binding between C-type lectin receptor, DC-SIGN, and Der p allergen.
The interaction between Dermatophagoides pteronyssinus (Der p) extracts and dendritic cell-specific intracellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN). (a) The expression and (b) percentages of DC-SIGN-positive cells in medium-MDDCs and Der p-MDDCs of nonallergic controls (NC) and allergic asthmatics (AC) were measured by flow cytometry. Data are expressed as mean fluorescence intensity (MFI) and percentages of total cells and analyzed by Student's t-test. (c) The lectin and calcium-dependent bindings between recombinant DC-SIGN and Der p extracts were performed in enzyme-linked immunosorbent assay (ELISA). Phenotypic analysis of monocyte-derived dendritic cells (MDDCs) incubated with or without Der p extracts.
Effect of Der p on the endocytotic capacity of immature MDDCs
The endocytotic capacity of MDDCs was measured based on the degree of uptake of fluorescein isothiocyanate (FITC)-coated beads assayed by flow cytometry. The representative analysis of endocytosis activity of MDDCs is shown in Figure 3, when incubated with FITC-coated beads from 10 min to 2 h at 37 °C (Figure 3a), and incubated with various concentrations of FITC-coated Der p for 1 h at 37 °C (Figure 3b). MDDCs exhibited greater endocytotic activity when incubated with Der p than in medium alone (P<0.01; Figure 3c), suggesting that Der p may enhance endocytosis in MDDCs, thereby favoring the immature phenotype of these cells. Moreover, we found the MDDCs obtained from allergic patients had significantly increased endocytotic activity compared with NC, both in medium-MDDCs (37.00±2.92 vs. 26.99±1.02, P<0.05) and Der p-MDDCs (38.37±2.76 vs. 28.26±2.01, P<0.05; Figure 3d).
Endocytosis capacity of medium-MDDCs and Der p-MDDCs. (a) Monocyte-derived dendritic cells (MDDCs) were incubated with fluorescein isothiocyanate (FITC)-coated beads from 10 min to 2 h at 37 °C. (b) MDDCs were incubated with various concentrations of FITC-coated Dermatophagoides pteronyssinus (Der p) for 1 h at 37 °C. Endocytosis capacity was measured by flow cytometry following washout of unbound beads. (c) The difference of endocytosis activity between medium-MDDCs and Der p-MDDCs when incubated with FITC-coated beads for 1 h at 37 °C. Data are analyzed by paired t-test. (d) The differences of endocytosis activity between nonallergic controls (NC) and allergic asthmatics (AS) in medium-MDDCs and Der p-MDDCs, respectively. Data were analyzed using Student's t-test and expressed as mean fluorescence intensity (MFI). *P<0.05; **P<0.01.
The decreased DC-SIGN was accompanied by endocytosis of Der p allergen
To understand how Der p allergen decreases DC-SIGN expression during differentiation of MDDCs, we considered multiple scenarios. First, we speculated that DC-SIGN mRNA is downregulated by Der p allergen; second, that Der p can cleave DC-SIGN by its protease activity; and third, that DC-SIGN on the cell surface can bind and endocytose Der p allergen into DC, thereby decreasing DC-SIGN expression at the plasma membrane. No significant differences in DC-SIGN mRNA levels were found between medium-MDDCs and Der p-MDDCs (Figure 4a) or between Der p allergic patients and controls (Figure 4b). In addition, heat inactivation of Der p protease activity did not affect DC-SIGN expression levels compared with MDDCs treated with active Der p (Figure 4c). In contrast, MDDCs incubated with FITC-conjugated Der p showed increased and overlapping internalization of Der p and DC-SIGN in the cytoplasm (Figure 4d). Together, these data indicate that Der p is endocytosed into DCs through DC-SIGN, thereby reducing DC-SIGN expression on the cell surface of MDDCs.
Dendritic cell-specific intracellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) mRNA expression levels in monocyte-derived dendritic cells (MDDCs). (a) The expressions of DC-SIGN and β-actin mRNA of MDDCs were analyzed by reverse transcriptase (RT)-PCR. Medium-MDDCs and Der p-MDDCs were collected from either nonallergic controls (NC) or from allergic asthmatics (AS). (b) The image data are represented by relative expressed quantity of DC-SIGN mRNA in medium-MDDCs and Der p-MDDCs of NC and AS. Data were analyzed by Student's t-test and are expressed as mean±s.e.m. (n=12 in each group). (c) The effect of Dermatophagoides pteronyssinus (Der p) and heat-inactivated Der p on surface DC-SIGN expression in immature dendritic cells (imDCs). These DCs were collected and analyzed for their cell surface DC-SIGN expressions by flow cytometry. Data were analyzed using Student's t-test and expressed as mean fluorescence intensity (MFI). *P<0.05. (d) The location of Der p and DC-SIGN in MMDCs. MMDCs were stimulated with or without fluorescein isothiocyanate (FITC)-Der p (green) for 2 h, and stained with anti-DC-SIGN antibody (red). Cells were imaged by confocal microscopy using a Leica TCS SP2 laser scanning confocal microscopy (n=8). Scale bar=5 μm. The color reproduction of this figure is available on the html full text version of the paper.
Effects of DC-SIGN ligands on the immature and mature (TNF-treated) MDDCs
Following stimulation with the DC-SIGN ligands, such as mannan, and Der p allergen, no detectable levels of the cytokines IL-6 or IL-12p70 were presented in culture supernatants of MDDCs (Figure 5), nor were there any changes in expression levels of the maturation markers CD80, CD86, and HLA-DR (data not shown). The lack of biological response in these experiments may be because of the immature status of MDDCs independent of DC-SIGN ligand stimulation. In contrast, there were significantly increased IL-6 and decreased IL-12p70 production of cytokines in the supernatants of lipopolysaccharide (LPS)-stimulated MDDCs collected from AS than those from NC (P<0.05). It is well known that inflammation in response to allergens is usually accompanied by proinflammatory cytokine production.19, 20 Therefore, maturation of MDDCs was triggered with the addition of recombinant human TNF (1,000 U ml–1), followed by stimulation with LPS, Der p, mannan, with and without anti-DC-SIGN blocking antibody, respectively (Figure 5). We also found that there were significantly increased IL-6 and decreased IL-12p70 cytokine production in LPS- and Der p-treated mature MDDCs collected from AS than those from NC (P<0.05). More importantly, anti-DC-SIGN antibody inhibited IL-6 and IL-12 production in Der p-treated MDDCs from AS and NC, but not in LPS-treated or mannan-treated MDDCs. There were also significantly increased IL-10 and decreased IL-12p70 production from MDDCs of AS than those from NC when MDDCs were stimulated with Der p allergen (Supplementary Figure S2 online).
Interleukin (IL)-12 and IL-6 cytokine production in the immature and matured (tumor necrosis factor-α (TNF-α) treated) monocyte-derived dendritic cells (MDDCs). The effect of lipopolysaccharide (LPS), mannan, and Dermatophagoides pteronyssinus (Der p) on the IL-12 and IL-6 cytokine production in the culture supernatant of immature and matured (TNF-α treated) MDDCs, isolated from nonallergic controls (NC; solid bars, n=19) and allergic asthmatics (AS; blank bars, n=14). Pretreatments with anti-DC-SIGN (dendritic cell-specific intracellular adhesion molecule-3-grabbing nonintegrin) blocking antibody for 6 h before LPS, mannan, and Der p stimulation in matured MDDCs were also performed. Data are expressed as means±s.e.m. and analyzed by paired t-test. *P<0.05; **P<0.01.
DC-SIGN ligand-treated MDDCs enhance Th2 polarity of CD4+ T cells
The allostimulatory capacity of MDDCs on naive CD4+ T cells was assayed by co-culture experiments using T-cell/DC ratios between 1:1 and 10:1. Messenger RNA for the Th1 (T-bet) and Th2 cytokine (GATA-3) transcription factors were assayed by reverse transcriptase-PCR. However, because mature MDDCs constitutively express T-bet (data not shown), only GATA-3 mRNA expression levels are presented here. There was significantly increased GATA-3 mRNA expression in CD4+ T cells co-cultured with Der p-MDDCs pretreated with Der p than with Der p-MDDCs pretreated with LPS (P<0.05). Moreover, adding ant-DC-SIGN antibody in the co-culture condition was able to inhibit GATA-3 mRNA expression in CD4+ T cells induced by Der p-MDDCs (P<0.05). There was no significant difference of GATA-3 mRNA expression in CD4+ T cells co-cultured with medium-MDDCs that were pretreated with LPS or Der p (Figure 6a).
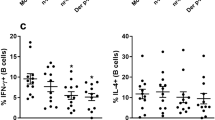
Cytokine production and GATA-3 mRNA expression in CD4+ T lymphocytes co-cultured with medium-MDDCs and Der p-MDDCS, respectively. Der p, Dermatophagoides pteronyssinus; MDDC, monocyte-derived dendritic cell. (b) The percentage ratio of interferon-γ (IFN-γ) and interleukin-4 (IL-4)-producing CD4+ T lymphocytes were stained for intracellular IFN-γ and IL-4 cytokines, respectively, and analyzed by flow cytometry. Data are analyzed by Student's t-test. *P<0.05. (a) mRNA levels for GATA-3 and β-actin were measured by reverse transcriptase (RT)-PCR in CD4+ T lymphocytes co-cultured with medium-MDDCs (n=17) and Der p-MDDCs (n=15) stimulated with lipopolysaccharide (LPS), Der p, or Der p with anti-DC-SIGN (dendritic cell-specific intracellular adhesion molecule-3-grabbing nonintegrin) blocking antibody for 24 h (DC/T cell=1:10).
To confirm the capacity for conditioned MDDCs to induce Th2 polarity, autologous naive T cells were co-cultured with medium-MDDCs and Der p-MDDCs for 10 days under various conditions. The relative percentage ratio of interferon-γ/IL-4-positive CD4+ T cells was significantly lower for cells cultured with medium-MDDCs and Der p-MDDCs pretreated with Der p than those with MDDCs pretreated with LPS (P<0.05) or Der p with anti-DC-SIGN blocking antibody (P<0.05, Figure 6b). These results indicate that DC-SIGN activation of MDDCs by Der p allergen promotes Th2-related cytokine production in co-cultured CD4+ T cells.
There was significant increased GATA-3 expression (P<0.05) as well as significantly decreased interferon-γ/IL-4 cytokine production ratio (P<0.05) in T cells when co-cultured with Der p-stimulated MDDCs of AS, but not with Der p-stimulated MDDCs of NC (Figure 7). This result clearly shows that the effects of Der p and change of DC-SIGN level in MDDCs of asthmatic patients had a Th2-dominant effect on T-cell cytokine production.
Cytokine production and GATA-3 mRNA expression in CD4+ T lymphocytes co-cultured with monocyte-derived dendritic cells (MDDCs) of allergic asthmatics (AS) and nonallergic controls (NC), respectively. (b) The percentage ratio of interferon-γ (IFN-γ) and interleukin-4 (IL-4)-producing CD4+ T lymphocytes were stained for intracellular IFN-γ and IL-4 cytokines, respectively, and analyzed by flow cytometry. Data are analyzed by Student's t-test. *P<0.05. (a) mRNA levels for GATA-3 and β-actin were measured by reverse transcriptase (RT)-PCR in CD4+ T lymphocytes co-cultured with MDDCs of AS (n=10) and NC (n=12) stimulated with lipopolysaccharide (LPS), Dermatophagoides pteronyssinus (Der p), or Der p with anti-DC-SIGN (dendritic cell-specific intracellular adhesion molecule-3-grabbing nonintegrin) blocking antibody for 24 h (DC/T cell=1:10).
Discussion
DCs are critical for the polarization of allergen-specific Th2 cells, leading to generation of Th2 cytokines that promote the key pathophysiological features of allergic reactions.21, 22 However, little is understood regarding how allergens induce differentiation and activation/maturation of mucosa DCs that subsequently lead to allergen-induced airway inflammation. Previously, we showed that Der p-induced nitric oxide and TNF-α production by activated alveolar macrophages occurs through binding and activation of CD14/TLR4 cell surface receptors.16, 17, 18, 19, 20 In this study, we show that, in vitro, DC-SIGN-bound Der p extracts were calcium dependent and inhibitable by maltose. In addition, despite differences of DC-SIGN expression levels and endocytosis capacity between MDDCs from AS and NC (Figures 2 and 3), Der p treatment resulted in decreased expression of DC-SIGN (Figure 2) as well as lower percentage of CD1a+CD14−-positive DCs during the differentiation process of immature MDDCs, regardless of Der p allergen sensitivity status (Figure 1). These results suggest the important role of C-type lectin receptor, DC-SIGN, in the determination of innate immune response of DCs when in contact with Der p allergen.
The ability of allergens to directly trigger the DC cytokine release and polarize immune response of naive CD4+ T cells is the key event for allergen-induced inflammatory diseases. During maturation of MDDCs by TNF-α treatment, we found Der p- as well as LPS-induced increased IL-6 (Th2-prone cytokine) and decreased IL-12 (Th1-prone cytokine) production in MDDCs obtained from AS than those from NC (Figure 5). Moreover, IL-6 and IL-12 production by Der-p-stimulated MDDCs are dependent on DC-SIGN activation, for anti-DC-SIGN antibodies block these cytokine productions, but not in LPS-stimulated MDDCs. The differences in cytokine productions between these two groups of MDDCs, although the mechanism is still not clear, may be because of lower expression of DC-SIGN proteins and more immature phenotypes of MDDCs in AS when compared with those of NC.
MDDCs from both groups pretreated with Der p allergen also exhibited increased polarization of the Th2 cytokine response of co-cultured naive CD4+ T cells in terms of increased levels of GATA-3 transcripts and decreased percentage ratio of interferon-γ/IL-4 cytokine producing cells (Figure 6). Recently, it is found that Ara h (Arachis hypogaea) 1, a major allergen of peanut, and soluble egg antigen of Schistosoma mansoni are able to polarize Th2 response via its interaction with DC-SIGN on MDDCs.23, 24 This interaction between allergens and DC-SIGN is suggested on the fucosylated glycan on allergens.25 Even the artificial DC-SIGN ligand of Lewisx-bovine serum albumin, a fucose-containing protein, elicited potent Th2 cytokine immune responses in mice that resulted from the inhibition of IL-12 production.26 Therefore, along with other reports, our study results suggest that the binding of DC-SIGN and the induced signaling events within DCs are associated with suppression of Th1 and/or enhancement of Th2 immunity.
Recently, mite allergens were reported to decrease the expression of DC-SIGN on the surface of DCs via activation of the inherent protease activity of Der p.18 It was speculated that decreased expression of DC-SIGN resulted in reduced binding of intercellular adhesion molecule-3, an endogenous DC-SIGN ligand for naive T cells, which decreased Th1 cytokine signaling.27, 28 Our results showed that Der p, either in the native or heat-inactivated state, decreased cell surface expression of DC-SIGN on MDDCs (Figure 4c). Therefore, the decreased expression of DC-SIGN in Der p-treated MDDCs is not dependent on the protease activity of Der p. We also found that the Der p/DC-SIGN complex is internalized into the cytosol of Der p-treated MDDCs, which may partly explain the downregulation of DC-SIGN after treatment with Der p allergen. Moreover, other evidence indicates that HDM may bind to DC-associated C-type lectin 2 (Dectin 2) leading to TLR-independent production of cytokines such as TNFα and IL-6 and activation of SYK (spleen tyrosine kinase) through FcγR to generate cysteinyl leukotrienes.17 Furthermore, a significant degree of crossregulation has been noted between CLRs and TLRs, and C-type lectin DC-SIGN is able to modulate Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor nuclear factor-κB, mimicking more closely the natural exposure to pathogens and allergens.29 These results suggest that decreased expression of DC-SIGN and/or interaction between allergen and CLRs on MDDCs may render its polarization activity on naive T cells into the Th2 cytokine response.
In summary, our observations of decreased expression of DC-SIGN and more immature phenotypes of MDDCs from Der p-sensitive asthmatic patients may partly explain the enhancement of the Th2 response associated with HDM-related allergies. In addition, Der p can modulate the differentiation and maturation of monocyte-derived DCs through the effect on DC-SIGN binding and downregulation of its expression, which may result in the change of polarization activity of DCs leading to the Th2 cytokine immune response. These results may provide a potential preventive and/or therapeutic modality to harness Der p allergen sensitization and allergen-induced airway inflammation.
Methods
Reagents. Der p extract (1 g lyophilized whole body extract in ether; Allergon, Engelholm, Sweden) was dissolved in pyogenic-free isotonic saline, filtered through a 0.22 μm filter, and stored at 70 °C before use. The LPS concentration of the preparations was <0.96 EU mg–1 of Der p (Limulus amebocyte lysate test; E-Toxate; Sigma-Aldrich, St Louis, MO). Recombinant human granulocyte-macrophage colony-stimulating factor, IL-4, and TNF-α were purchased from Peprotech EC (London, UK).
Study subjects. The study population consisted of 20 mite (Der p)-sensitive allergic asthmatics (mean age, 11.2 years) and 20 age-matched non-atopic controls. The definition of asthma was based on guidelines instituted by the GINA (Global Initiative for Asthma). Sensitivity to the HDM, Der p, was evaluated by a skin prick test and reactivity to a specific immunoglobulin E antibody to Der p using the Unicap system (Pharmacia Diagnostics, Uppsala, Sweden). Age-matched non-atopic controls did not have any of the above allergic asthmatic symptoms in their previous physical check-ups and yielded negative results in the skin prick test and immunoglobulin E to Der p. This study was approved by the human research committee of the National Cheng Kung University Hospital, and informed consent was obtained from all subjects or their guardians.
Solid-phase binding assay . Microplates were first coated with varying concentrations of Der p extract and bovine serum albumin in 0.05 M carbonate–bicarbonate buffer (pH 9.6) and blocked with assay buffer (10 mM Tris-Cl, 50 mM CaCl2, 150 mM NaCl, and 2.5% goat serum). The plates were then incubated with 0.5 μg ml–1 of recombinant human DC-SIGN (R&D Systems, Minneapolis, MN) at room temperature for 2 h followed by incubation with peroxidase-conjugated goat anti-human immunoglobulin G Fc antibodies (Pierce, Rockford, IL) for 1 h at room temperature and by the addition of a substrate, tetramethylbenzidine (R&D Systems). The reactions were stopped by the addition of 2 N H2SO4 and read in a microplate reader. The relative binding activity was expressed as optical density for each test antigen after subtracting the values from the background.
Generation of MDDCs. CD14+ monocytes obtained from peripheral blood mononuclear cells in study subjects were purified with the monocyte separation kit II and magnetic-activated cell sorting columns (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocol. Purity was assessed by staining with anti-CD14 FITC antibody (Becton Dickinson, Franklin Lakes, NJ) and was routinely found to be >90%. These cells were washed with RPMI-1640 medium and cultured in 24-well flat-bottomed cultured plates in the presence of 800 U recombinant human granulocyte-macrophage colony-stimulating factor and 1,000 U recombinant human IL-4 in RPMI-1640 medium supplemented with 2 μM glutamine, 50 μM 2-Mercaptomethanol, 1 μM sodium pyruvate, 1 μM non-essential amino acids, 1% Pen/Strep, and 10 % FBS (GIBCO, Grand Island, NY). Medium was replaced on days 3 and 5 of culture, and on day 7, the immature DCs were treated with different concentrations of Der p extracts for 24 h. The resulting cells exhibited DC morphology and clustering, and were uniformly CD14−, CD1a+, CD83low, and HLA-DR+. To induce maturation, recombinant human TNF-α (1,000 U ml–1) was added on day 8, and the mature MDDCs were treated with different concentrations of LPS, mannan, or Der p for another 24 h. For blocking the allergen-mediated effect through DC-SIGN, cells were cultured in the presence of anti-DC-SIGN blocking antibodies (1 μg ml–1, R&D Systems) for 1 h before the stimulation of the MDDCs.
Flow cytometric analysis of cell surface molecules. Phenotypic analysis of surface markers was then performed by staining with fluorochrome-conjugated monoclonal antibodies to CD14, CD1a, CD11c, HLA-DR, DC-SIGN (CD209), and appropriate isotype-matched antibodies according to the manufacturer's protocols (Becton Dickinson). The cells were washed, fixed in 1% formaldehyde, and analyzed on a flow cytometer (FACSort, Becton Dickinson). Results were expressed as the relative fluorescence intensity (rFI), calculated as rFI=(MFI sample–MFI control)/MFI control, where MFI is the mean fluorescence intensity.
Endocytosis activity of DCs. MDDCs in 2 × 105 cells per well were incubated at 37 or 4 °C in media containing 10 μg ml–1 of FITC-labeled latex beads (FITC beads, 1 ìm diameter, Polyscience, Warrington, PA) for 1 h, and then washed and fixed in cold 1% formalin. The uptake of FITC beads was quantitated by fluorescence-activated cell sorting.
Confocal microscopy. Der p was labeled with FITC according to EZ-Label FITC Protein Labeling Kit (Pierce). Immature DCs were incubated with FITC-conjugated Der p for 2 h at 37 °C, and fixed cells were stained with DC-SIGN monoclonal antibody and tetramethyl rhodamine isothiocyanate-conjugated secondary antibody. Cells were imaged with a Leica (Wetzlar, Germany) TCS SP2 laser scanning confocal microscope.
DC–T-cell co-culture assay. CD4+ T cells were purified from peripheral blood mononuclear cells with a naive CD4+ T-cell isolation kit and magnetic-activated cell sorting columns (Miltenyi Biotec). After 7 days of culture with IL-4 and granulocyte-macrophage colony-stimulating factor, immature DCs were stimulated with DC-SIGN ligands following treatment with LPS (1 μg ml–1) for 6 h and then co-cultured with autologous purified naive CD4+ T cells in the ratio of 1:1 or 1:10 (DC/T cell). On day 5, cultures were supplemented with IL-2 (10 U ml–1) as necessary. On day 10, the cells were stimulated with 50 ng ml–1 phorbol-12-myristate-13-acetate (Sigma-Aldrich) and 10 μg ml–1 calcium ionophore A23187 (Sigma-Aldrich) in the presence of 10 μg ml–1 Brefeldin A (Sigma-Aldrich) for accumulation of intracellular cytokines. Then, these cells were harvested and fixed with eBioscience IC fixation buffer (eBioscience, San Diego, CA) and stained for anti-IL-4 and anti-interferon-γ antibody (BD PharMingen, Oxford, UK) to measure the intracellular cytokine production in T cells.
Quantification of cytokines. The effects of Der p extract on the production of IL-6 and IL-12p70 cytokines during the differentiation and maturation process of MDDCs (2 × 105) were measured by appropriate enzyme-linked immunosorbent assay kits (R&D Systems).
Reverse transcriptase-PCR for DC-SIGN and GATA-3 mRNA expression. The total mRNA expression of DC-SIGN (CD209) in MDDCs and GATA-3 in CD4+ T cells co-cultured with MDDCs was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA). Reverse transcriptase-PCR products were analyzed on a 2% agarose gel (Promega, Madison, WI).
Statistical analysis. Data are shown as mean±s.e.m. and were analyzed by a two-tailed Mann–Whitney U-test using GraphPad Prism 4.0 (GraphPad, San Diego, CA). The P-values of <0.05 were regarded as significant.
References
Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1988).
van Rijt, L.S. & Lambrecht, B.N. Dendritic cells in asthma: a function beyond sensitization. Clin. Exp. Allergy 35, 1125–1134 (2005).
Vermaelen, K. & Pauwels, R. Accelerated airway dendritic cell maturation, trafficking, and elimination in a mouse model of asthma. Am. J. Respir. Cell Mol. Biol. 29, 405–409 (2003).
Figdor, C.G., van Kooyk, Y. & Adema, G.J. C-type lectin receptors on dendritic cells and Langerhans cells. Nat. Rev. Immunol. 2, 77–84 (2002).
Cambi, A. & Figdor, C.G. Dual function of C-type lectin-like receptors in the immune system. Curr. Opin. Cell Biol. 15, 539–546 (2003).
Kalinski, P., Hilkens, C.M., Wierenga, E.A. & Kapsenberg, M.L. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol. Today 20, 561–567 (1999).
Kapsenberg, M.L. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 3, 984–993 (2003).
Allakhverdi, Z., Bouguermouh, S., Rubio, M. & Delespesse, G. Adjuvant activity of pollen grains. Allergy 60, 1157–1164 (2005).
Traidl-Hoffmann, C. et al. Pollen-associated phytoprostanes inhibit dendritic cell interleukin-12 production and augment T helper type 2 cell polarization. J. Exp. Med. 201, 627–636 (2005).
Hammad, H. et al. Th2 polarization by Der p 1–pulsed monocyte-derived dendritic cells is due to the allergic status of the donors. Blood 98, 1135–1141 (2001).
Hammad, H. et al. Monocyte-derived dendritic cells exposed to Der p 1 allergen enhance the recruitment of Th2 cells: major involvement of the chemokines TARC/CCL17 and MDC/CCL22. Eur. Cytokine Netw. 14, 219–228 (2003).
Ghaemmaghami, A.M., Gough, L., Sewell, H.F. & Shakib, F. The proteolytic activity of the major dust mite allergen Der p 1 conditions dendritic cells to produce less interleukin-12: allergen-induced Th2 bias determined at the dendritic cell level. Clin. Exp. Allergy 32, 1468–1475 (2002).
Chen, C.L., Lee, C.T., Liu, Y.C., Wang, J.Y., Lei, H.Y. & Yu, C.K. House dust mite Dermatophagoides farinae augments proinflammatory mediator productions and accessory function of alveolar macrophages: implications for allergic sensitization and inflammation. J. Immunol. 170, 528–536 (2003).
Yu, C.K. & Chen, C.L. Activation of mast cells is essential for development of house dust mite Dermatophagoides farinae-induced allergic airway inflammation in mice. J. Immunol. 171, 3808–3815 (2003).
Hammad, H., Chieppa, M., Perros, F., Willart, M.A., Germain, R.N. & Lambrecht, B.N. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 15, 410–416 (2009).
Liu, C.F. et al. Mite allergen induces nitric oxide production in alveolar macrophages via the CD14/TLR4 complex, and is inhibited by surfactant protein D. Clin. Exp. Allergy 35, 1615–1624 (2005).
Barrett, N.A., Maekawa, A., Rahman, O.M., Austen, K.F. & Kanaoka, Y. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J. Immunol. 182, 1119–1128 (2009).
Furmonaviciene, R. et al. The protease allergen Der p 1 cleaves cell surface DC-SIGN and DC-SIGNR: experimental analysis of in silico substrate identification and implications in allergic responses. Clin. Exp. Allergy 37, 231–242 (2007).
Rubio, M.T. et al. Maturation of human monocyte-derived dendritic cells (MoDCs) in the presence of prostaglandin E2 optimizes CD4 and CD8 T cell-mediated responses to protein antigens: role of PGE2 in chemokine and cytokine expression by MoDCs. Int. Immunol. 12, 1561–1572 (2005).
Liu, C.F., Rivere, M., Huang, H.J., Puzo, G. & Wang, J.Y. Surfactant protein D inhibits mite-induced alveolar macrophage and dendritic cell activations through TLR signaling and DC-SIGN expression. Clin. Exp. Allergy 40, 111–122 (2010).
Lambrecht, B.N., De Veerman, M., Coyle, A.J., Gutierrez-Ramos, J.C., Thielemans, K. & Pauwels, R.A. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J. Clin. Invest. 106, 551–559 (2000).
MacDonald, A.S. & Maizels, R.M. Alarming dendritic cells for Th2 induction. J. Exp. Med. 205, 13–17 (2008).
Shreffler, W.G. et al. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J. Immunol. 177, 3677–3685 (2006).
van Liempt, E. et al. Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C-type lectins and suppress TLR-induced dendritic cell activation. Mol. Immunol. 44, 2605–2615 (2007).
Hsu, S.C. et al. Functional interaction of common allergens and a C-type lectin receptor, dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN), on human dendritic cells. J. Biol. Chem. 285, 7903–7910 (2010).
Hsu, S.C. et al. Antigen coupled with Lewis-x trisaccharides elicits potent immune responses in mice. J. Allergy Clin. Immunol. 119, 1522–1528 (2007).
Caparrós, E. et al. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. Blood 107, 3950–3958 (2006).
Bleijs, D.A., de Waal-Malefyt, R., Figdor, C.G. & van Kooyk, Y. Co-stimulation of T cells results in distinct IL-10 and TNF-alpha cytokine profiles dependent on binding to ICAM-1, ICAM-2 or ICAM-3. Eur. J. Immunol. 29, 2248–2258 (1999).
Gringhuis, S.I., Dunnen, J.D., Litjens, M., van het Hof, B., van Kooyk, Y. & Geijtenbeek, T.B. C-type lectin DC-SIGN modulates toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kB. Immunity 26, 605–616 (2007).
Acknowledgements
This study was supported by the grant NSC 98-2314-B-006-048-MY3 from National Science Council, Taiwan.
Author ContributionsJ.Y.W designed the research, analyzed the data, interpreted results, and wrote the paper; H.Y.H. performed the studies, analyzed the data, and interpreted results. Y.L.L and C.F.L. provided the necessary help and technological support for experiments in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Huang, HJ., Lin, YL., Liu, CF. et al. Mite allergen decreases DC-SIGN expression and modulates human dendritic cell differentiation and function in allergic asthma. Mucosal Immunol 4, 519–527 (2011). https://doi.org/10.1038/mi.2011.17
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2011.17
This article is cited by
-
C-Type Lectin Receptor Mediated Modulation of T2 Immune Responses to Allergens
Current Allergy and Asthma Reports (2023)
-
Modulating local airway immune responses to treat allergic asthma: lessons from experimental models and human studies
Seminars in Immunopathology (2020)
-
Lactobacillus gasseri attenuates allergic airway inflammation through PPARγ activation in dendritic cells
Journal of Molecular Medicine (2018)
-
Critical role of IL-6 in dendritic cell-induced allergic inflammation of asthma
Journal of Molecular Medicine (2016)
-
Innate immunostimulatory properties of allergens and their relevance to food allergy
Seminars in Immunopathology (2012)