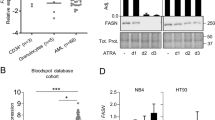
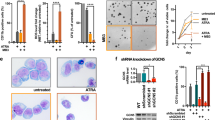
Abstract
Use of all-trans retinoic acid (ATRA) as a differentiation agent has been limited to acute promyelocytic leukemia (APL) as non-APL leukemias are insensitive to ATRA. We recently demonstrated that the rexinoid, bexarotene, induces differentiation and therapeutic responses in patients with refractory AML. Rexinoids bind and activate retinoid X receptors (RXRs); however, rexinoids alone are incapable of activating retinoic acid receptor (RAR)/RXR complexes, suggesting that myeloid differentiation can occur independent of RAR. In this study, we demonstrate that rexinoid differentiation of AML cells is RAR independent and requires the expression of PU.1. Because of the promiscuousness of RXR with other nuclear receptors, myeloid differentiation by bexarotene with other nuclear receptor ligands was explored. Bexarotene cooperated with ATRA to enhance differentiation in some AML cell lines; however, the combination of bexarotene with the PPARγ agonist rosiglitazone did not. In contrast, bexarotene combined with liver X receptor (LXR) agonists, T0901317 or GW3965, induced potent differentiation and cytotoxicity in AML cell lines and primary human AML cells, but not in normal progenitor cells. These results suggest that RXR/LXR-regulated gene expression in normal cells is deregulated in AML cells and identifies a potential role for these agonists in differentiation therapy of non-APLs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ablain J, de The H . Revisiting the differentiation paradigm in acute promyelocytic leukemia. Blood 2011; 117: 5795–5802.
Alcalay M, Zangrilli D, Pandolfi PP, Longo L, Mencarelli A, angelo G et al. Translocation breakpoint of acute promyelocytic leukemia lies within the retinoic acid receptor {alpha} locus. Proc Natl Acad Sci USA 1991; 88: 1977–1981.
Juliusson G, Karlsson K, Lazarevic V, Wahlin A, Brune M, Antunovic P et al. Hematopoietic stem cell transplantation rates and long-term survival in acute myeloid and lymphoblastic leukemia: real-world population-based data from the Swedish Acute Leukemia Registry 1997–2006. Cancer 2011; 117: 4238–4246.
Tsai DE, Luger SM, Andreadis C, Vogl DT, Kemner A, Potuzak M et al. A phase I study of bexarotene, a retinoic X receptor agonist, in non-M3 acute myeloid leukemia. Clin Cancer Res 2008; 14: 5619–5625.
Aranda A, Pascual A . Nuclear hormone receptors and gene expression. Physiol Rev 2001; 81: 1269–1304.
Kizaki M, Dawson MI, Heyman R, Elster E, Morosetti R, Pakkala S et al. Effects of novel retinoid X receptor-selective ligands on myeloid leukemia differentiation and proliferation in vitro. Blood 1996; 87: 1977–1984.
Altucci L, Rossin A, Hirsch O, Nebbioso A, Vitoux D, Wilhelm E et al. Rexinoid-triggered differentiation and tumor-selective apoptosis of acute myeloid leukemia by protein kinase A-mediated desubordination of retinoid X receptor. Cancer Res 2005; 65: 8754–8765.
Mueller BU, Pabst T, Fos J, Petkovic V, Fey MF, Asou N et al. ATRA resolves the differentiation block in t(15;17) acute myeloid leukemia by restoring PU.1 expression. Blood 2006; 107: 3330–3338.
Rosenbauer F, Wagner K, Kutok JL, Iwasaki H, Le Beau MM, Okuno Y et al. Acute myeloid leukemia induced by graded reduction of a lineage-specific transcription factor, PU.1. Nat Genet 2004; 36: 624–630.
Vangala RK, Heiss-Neumann MS, Rangatia JS, Singh SM, Schoch C, Tenen DG et al. The myeloid master regulator transcription factor PU.1 is inactivated by AML1-ETO in t(8;21) myeloid leukemia. Blood 2003; 101: 270–277.
Mueller BU, Pabst T, Osato M, Asou N, Johansen LM, Minden MD et al. Heterozygous PU.1 mutations are associated with acute myeloid leukemia. Blood 2003; 101: 2074.
Dahl R, Walsh JC, Lancki D, Laslo P, Iyer SR, Singh H et al. Regulation of macrophage and neutrophil cell fates by the PU.1:C/EBPalpha ratio and granulocyte colony-stimulating factor. Nat Immunol 2003; 4: 1029–1036.
Walsh JC, DeKoter RP, Lee HJ, Smith ED, Lancki DW, Gurish MF et al. Cooperative and antagonistic interplay between PU.1 and GATA-2 in the specification of myeloid cell fates. Immunity 2002; 17: 665–676.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods (San Diego, CA 2001; 25: 402–408.
Chen Q, Ross AC . Retinoic acid regulates cell cycle progression and cell differentiation in human monocytic THP-1 cells. Exp Cell Res 2004; 297: 68–81.
Iijima K, Honma Y, Niitsu N . Granulocytic differentiation of leukemic cells with t(9;11)(p22;q23) induced by all-trans-retinoic acid. Leuk Lymphoma 2004; 45: 1017–1024.
Tagliafico E, Tenedini E, Manfredini R, Grande A, Ferrari F, Roncaglia E et al. Identification of a molecular signature predictive of sensitivity to differentiation induction in acute myeloid leukemia. Leukemia 2006; 20: 1751–1758.
Howell SR, Shirley MA, Grese TA, Neel DA, Wells KE, Ulm EH . Bexarotene metabolism in rat, dog, and human, synthesis of oxidative metabolites, and in vitro activity at retinoid receptors. Drug Metab Dispos 2001; 29: 990–998.
Perez E, Bourguet W, Gronemeyer H, de Lera AR . Modulation of RXR function through ligand design. Biochim Biophys Acta 2011; 1821: 57–69.
Shiohara M, Dawson MI, Hobbs PD, Sawai N, Higuchi T, Koike K et al. Effects of novel RAR- and RXR-selective retinoids on myeloid leukemic proliferation and differentiation in vitro. Blood 1999; 93: 2057–2066.
van de Merbel NC, van Veen JH, Wilkens G, Loewen G . Validated liquid chromatographic method for the determination of bexarotene in human plasma. Chromatogr 2002; 775: 189–195.
Kizaki M, Ueno H, Matsushita H, Takayama N, Muto A, Awaya N et al. Retinoid resistance in leukemic cells. Leuk Lymphoma 1997; 25: 427–434.
Park DJ, Chumakov AM, Vuong PT, Chih DY, Gombart AF, Miller WH Jr et al. CCAAT/enhancer binding protein epsilon is a potential retinoid target gene in acute promyelocytic leukemia treatment. J Clin invest 1999; 103: 1399–1408.
Lekstrom-Himes JA . The role of C/EBP(epsilon) in the terminal stages of granulocyte differentiation. Stem cells (Dayton, OH) 2001; 19: 125–133.
Friedman AD . Transcriptional control of granulocyte and monocyte development. Oncogene 2007; 26: 6816–6828.
Yoshida H, Ichikawa H, Tagata Y, Katsumoto T, Ohnishi K, Akao Y et al. PML-retinoic acid receptor alpha inhibits PML IV enhancement of PU.1-induced C/EBPepsilon expression in myeloid differentiation. Mol Cell Biol 2007; 27: 5819–5834.
Kueh HY, Rothenberg EV . Regulatory gene network circuits underlying T cell development from multipotent progenitors. Wiley Interdiscip Rev Syst Biol Med 2011; 4: 79–102.
Novershtern N, Subramanian A, Lawton LN, Mak RH, Haining WN, McConkey ME et al. Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell 2011; 144: 296–309.
Konopleva M, Elstner E, McQueen TJ, Tsao T, Sudarikov A, Hu W et al. Peroxisome proliferator-activated receptor gamma and retinoid X receptor ligands are potent inducers of differentiation and apoptosis in leukemias. Mol Cancer Ther 2004; 3: 1249–1262.
Kohro T, Nakajima T, Wada Y, Sugiyama A, Ishii M, Tsutsumi S et al. Genomic structure and mapping of human orphan receptor LXR alpha: upregulation of LXRa mRNA during monocyte to macrophage differentiation. J Atheroscl Thromb 2000; 7: 145–151.
Peeters SD, van der Kolk DM, de Haan G, Bystrykh L, Kuipers F, de Vries EG et al. Selective expression of cholesterol metabolism genes in normal CD34+CD38− cells with a heterogeneous expression pattern in AML cells. Exp Hemat 2006; 34: 622–630.
Kumar N, Solt LA, Conkright JJ, Wang Y, Istrate MA, Busby SA et al. The benzenesulfoamide T0901317 [N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluorometh yl)ethyl]phenyl]-benzenesulfonamide] is a novel retinoic acid receptor-related orphan receptor-alpha/gamma inverse agonist. Mol Pharmacol 2010; 77: 228–236.
Solt LA, Kumar N, Nuhant P, Wang Y, Lauer JL, Liu J et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 2011; 472: 491–494.
Collins SJ . The role of retinoids and retinoic acid receptors in normal hematopoiesis. Leukemia 2002; 16: 1896–1905.
Gallagher RE . Retinoic acid resistance in acute promyelocytic leukemia. Leukemia 2002; 16: 1940–1958.
Kastner P, Lawrence HJ, Waltzinger C, Ghyselinck NB, Chambon P, Chan S . Positive and negative regulation of granulopoiesis by endogenous RARalpha. Blood 2001; 97: 1314–1320.
Ricote M, Snyder CS, Leung HY, Chen J, Chien KR, Glass CK . Normal hematopoiesis after conditional targeting of RXRalpha in murine hematopoietic stem/progenitor cells. J Leuk Biol 2006; 80: 850–861.
Durual S, Rideau A, Ruault-Jungblut S, Cossali D, Beris P, Piguet V et al. Lentiviral PU.1 overexpression restores differentiation in myeloid leukemic blasts. Leukemia 2007; 21: 1050–1059.
Szanto A, Nagy L . Retinoids potentiate peroxisome proliferator-activated receptor gamma action in differentiation, gene expression, and lipid metabolic processes in developing myeloid cells. Mol Pharmacol 2005; 67: 1935–1943.
Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM . Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 1998; 93: 229–240.
Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM . PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 1998; 93: 241–252.
Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 2010; 328: 1689–1693.
Acknowledgements
We thank the Stem Cell and Xenograft Core, the Human Immunology Core (Philadelphia, PA, USA), the Flow Cytometry Core, the Bioinformatics Core at the Perelman School of Medicine, UPENN Cancer Center and the Carroll lab for advice and assistance. This study was supported by K01-CA-129151 (PVS), 1R01CA149566 (MC), the Perelman School of Medicine at the University of Pennsylvania and the US Veterans Administration (MC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
MC receives research support from Glaxo Smith Kline, Sanofi Aventis Corporation and Tetralogic Pharmaceuticals.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Sanchez, P., Glantz, S., Scotland, S. et al. Induced differentiation of acute myeloid leukemia cells by activation of retinoid X and liver X receptors. Leukemia 28, 749–760 (2014). https://doi.org/10.1038/leu.2013.202
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2013.202
Keywords
This article is cited by
-
Selective Inhibition of JAK1 Primes STAT5-Driven Human Leukemia Cells for ATRA-Induced Differentiation
Targeted Oncology (2021)
-
A distinct epigenetic program underlies the 1;7 translocation in myelodysplastic syndromes
Leukemia (2019)
-
Novel strategies for targeting leukemia stem cells: sounding the death knell for blood cancer
Cellular Oncology (2017)
-
Targeting liver X receptors in cancer therapeutics
Nature Reviews Cancer (2015)