Abstract
Objective:
Leptin is an adipokine that regulates energy homeostasis. The objective of this study was to establish a gestational age-specific standard for amniotic fluid leptin (AFL) levels and examine the relationship between AFL, maternal overweight and fetal growth restriction.
Study Design:
Amniotic fluid was obtained at mid-gestation from singleton gravidas, and leptin was quantified using enzyme-linked immunosorbent assay. Amniotic fluid samples from 321 term pregnancies were analyzed. Clinical data, including fetal ultrasound measurements and maternal and infant characteristics, were available for a subset of patients (n=45).
Results:
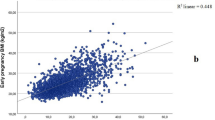
The median interquartile range AFL level was significantly higher at 14 weeks’ gestation (2133 pg ml−1 (1703 to 4347)) than after 33 weeks’ gestation (519 pg ml−1 (380 to 761), P trend<0.0001), an average difference of 102 pg ml−1 per week. AFL levels were positively correlated with maternal pre-pregnancy body mass index (BMI) (r=0.36, P=0.03) adjusting for gestational age at measurement, but were not associated with fetal growth.
Conclusions:
AFL levels are higher at mid-gestation than at late gestation, and are associated with maternal pre-pregnancy BMI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Bernstein I, Gabbe SG et al. Intrauterine growth restriction In: Gabbe SG, Niebyl JR, Simpson JL, Annas GJ (eds). Obstetrics: Normal and Problem Pregnancies 6th edn Elsevier: New York, NY, USA, 2012 pp 857–865.
Creasy RK, Resnik R . Intrauterine growth restriction In: Creasy RK, Resnik R (eds). Maternal-Fetal Medicine: Principles and Practice 4th edn. Saunders: Philadelphia, PA, USA, 2004 pp 495–512.
Alkalay AL, Graham JM, Pomerance JJ . Evaluation of neonates born with intrauterine growth retardation: review and practice guidelines. J Perinatol 1998; 18: 142–151.
McIntire DD, Bloom SL, Casey BM, Leveno KJ . Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med 1999; 340: 1234–1238.
Godfrey KM, Barker DJ . Fetal programming and adult health. Public Health Nutr 2001; 4: 611–624.
Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM . Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 1993; 36: 62–67.
Neerhof MG . Causes of intrauterine growth restriction. Clin Perinatol 1995; 22: 375–385.
Szentpéteri I, Rab A, Kornya L, Kovács P, Joó JG . Gene expression patterns of vascular endothelial growth factor (VEGF-A) in human placenta from pregnancies with intrauterine growth restriction. J Matern Fetal Neonatal Med 2013; 26: 984–989.
Vrachnis N, Kalampokas E, Sifakis S, Vitoratos N, Kalampokas T, Botsis D et al. Placental growth factor (PlGF): a key to optimizing fetal growth. J Matern Fetal Neonatal Med 2013; 26: 995–1002.
Briana DD, Malamitsi-Puchner A . Intrauterine growth restriction and adult disease: the role of adipocytokines. Eur J Endocrinol 2009; 160: 337–347.
Ornoy A . Biomarkers of maternal diabetes and its complication in pregnancy. Reprod Toxicol 2012; 34: 174–179.
Ahima RS, Flier JS . Leptin. Annu Rev Physiol 2000; 62: 413–437.
Popovic V, Casanueva FF . Leptin, nutrition and reproduction: new insights. Hormones 2002; 1: 204–217.
Senaris R, Garcia-Caballero T, Casabiell X, Gallego R, Castro R, Considine RV et al. Synthesis of leptin in human placenta. Endocrinology 1997; 138: 4501–4504.
Lepercq J, Challier JC, Guerre-Millo M, Cauzac M, Vidal H, Hauguel-de Mouzon S . Prenatal leptin production: evidence that fetal adipose tissue produces leptin. J Clin Endocrinol Metab 2001; 86: 2409–2413.
Rasmussen KM, Yaktine AL (eds). Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. National Academies Press (US): Washington (DC), CO, USA, 2009.
Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS . New intrauterine growth curves based on United States data. Pediatrics 2010; 125: e214–e224.
Alexopoulos A, Bravou V, Peroukides S, Kaklamanis L, Varakis J, Alexopoulos D et al. Bone regulatory factors NFATc1 and Osterix in human calcific aortic valves. Int J Cardiol 2010; 139: 142–149.
Cole TJ, Green PJ . Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 1992; 11: 1305–1319.
Henson MC, Castracane VD . Leptin in pregnancy: an update. Biol Reprod 2006; 74: 218–229.
Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H et al. Nonadipose tissue production of leptin: leptin as a novel placenta derived hormone in humans. Nat Med 1997; 3: 1029–1033.
Karakosta P, Chatzi L, Plana E, Margioris A, Castanas E, Kogevinas M . Leptin levels in cord blood and anthropometric measures at birth: a systematic review and meta-analysis. Paediatr Perinat Epidemiol 2011; 25: 150–163.
Chan TF, Su JH, Chung YF, Hsu YH, Yeh YT, Yuan SS . Elevated amniotic fluid leptin levels in pregnant women who are destined to develop preeclampsia. Acta Obstet Gynecol Scand 2006; 85: 171–174.
Cagnacci A, Arangino S, Caretto S, Mazza V, Volpe A . Sexual dimorphism in the levels of amniotic fluid leptin in pregnancies at 16 weeks of gestation: relation to fetal growth. Eur J Obstet Gynecol Reprod Biol 2006; 124: 53–57.
Tessier DR, Ferraro ZM, Gruslin A . Role of leptin in pregnancy: Consequences of maternal obesity. Placenta 2013; 34: 205–211.
Oktem O, Dedeoğlu N, Oymak Y, Sezen D, Köksal L, Pekin T et al. Maternal serum, amniotic fluid and cord leptin levels at term: their correlations with fetal weight. J Perinat Med 2004; 32: 266–271.
Lea RG, Howe D, Hannah LT, Bonneau O, Hunter L, Hoggard N . Placental leptin in normal, diabetic and fetal growth-retarded pregnancies. Mol Hum Reprod 2000; 6: 763–769.
Lappas M, Permezel M, Rice GE . Leptin and adiponectin stimulate the release of proinflammatory cytokines and prostaglandins from human placenta and maternal adipose tissue via nuclear factor-kappaB, peroxisomal proliferator-activated receptor-gamma and extracellularly regulated kinase 1/2. Endocrinology 2005; 146: 3334–3342.
Misra VK, Trudeau S . The influence of overweight and obesity on longitudinal trends in maternal serum leptin levels during pregnancy. Obesity 2011; 19: 416e21.
Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S . Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 2009; 32: 1076e80.
Alexe DM, Syridou G, Petridou ET . Determinants of early life leptin levels and later life degenerative outcomes. Clinl Med Res 2006; 4: 326–335.
Nagy GR, Gyõrffy B, Galamb O, Molnár B, Nagy B, Papp Z . Use of routinely collected amniotic fluid for whole-genome expression analysis of polygenic disorders. Clin Chem 2006; 52: 2013–2020.
Misra VK, Straughen JK, Trudeau S . Maternal serum leptin during pregnancy and infant birth weight: the influence of maternal overweight and obesity. Obesity (Silver Spring) 2013; 21: 1064–1069.
Molvarec A, Szarka A, Walentin S, Beko G, Karádi I, Prohászka Z et al. Serum leptin levels in relation to circulating cytokines, chemokines, adhesion molecules and angiogenic factors in normal pregnancy and preeclampsia. Reprod Biol Endocrinol 2011; 9: 124.
Bloomfield FH, Oliver MH, Harding JE . The late effects of fetal growth patterns. Arch Dis Child Fetal Neonatal Ed 2006; 91: F299–F304.
McCormack RT, Armstrong J, Leonard D . Codevelopment of genome-based therapeutics and companion diagnostics: insights from an Institute of Medicine roundtable. JAMA 2014; 311: 1395–1396; Erratum in: JAMA 2014; 311: 1396.
Acknowledgements
We thank Michelle Faust (Heart Institute Research Core) and Peggy Walsh (Hatton Research Center) for their assistance. Funding. We disclosed receipt of the following financial support for the research, authorship and/or publication of this article: the Cincinnati Children’s Research Foundation (RBH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Journal of Perinatology website
Rights and permissions
About this article
Cite this article
Scott-Finley, M., Woo, J., Habli, M. et al. Standardization of amniotic fluid leptin levels and utility in maternal overweight and fetal undergrowth. J Perinatol 35, 547–552 (2015). https://doi.org/10.1038/jp.2015.39
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2015.39