Abstract
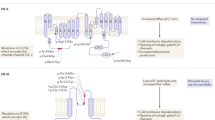
Primary aldosteronism is the most common form of endocrine hypertension. This disorder comprises both sporadic and familial forms. Four familial forms of primary aldosteronism (FH-I to FH-IV) have been described. FH-III is caused by germline mutations in KCNJ5, encoding the potassium channel Kir3.4 (also called GIRK4). These mutations alter the selectivity filter of the channel and lead to abnormal ion currents with loss of potassium selectivity, sodium influx and consequent increased intracellular calcium that causes excessive aldosterone biosynthesis. To date, eleven families have been reported, carrying six different mutations. Although the clinical features are variable, FH-III patients often display severe hyperaldosteronism with an early onset, associated with hypokalemia and diabetes insipidus-like symptoms. In most cases FH-III patients are resistant to pharmacological therapy and require bilateral adrenalectomy to control symptoms. In the present manuscript, we review the genetics and pathological basis of FH-III, the diagnostic work-up, clinical features and therapeutic management. Finally, we will describe a new case of FH-III of an Italian patient carrying a Gly151Arg mutation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Käyser SC, Dekkers T, Groenewoud HJ, van der Wilt GJ, Carel Bakx J, van der Wel MC et al. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J Clin Endocrinol Metab 2016; 101: 2826–2835.
Savard S, Amar L, Plouin P-F, Steichen O . Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension 2013; 62: 331–336.
Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A et al. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab 2013; 98: 4826–4833.
Mulatero P, Milan A, Williams TA, Veglio F . Mineralocorticoid receptor blockade in the protection of target organ damage. Cardiovasc Hematol Agents Med Chem 2006; 4: 75–91.
Monticone S, Else T, Mulatero P, Williams TA, Rainey WE . Understanding primary aldosteronism: impact of next generation sequencing and expression profiling. Mol Cell Endocrinol 2015; 399: 311–320.
Korah HE, Scholl UI . An update on familial hyperaldosteronism. Horm Metab Res 2015; 47: 941–946.
Lifton RP, Dluhy RG, Powers M, Rich GM, Cook S, Ulick S et al. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature 1992; 355: 262–265.
Mulatero P, Curnow KM, Aupetit-Faisant B, Foekling M, Gomez-Sanchez C, Veglio F et al. Recombinant CYP11B genes encode enzymes that can catalyze conversion of 11-deoxycortisol to cortisol, 18-hydroxycortisol, and 18-oxocortisol. J Clin Endocrinol Metab 1998; 83: 3996–4001.
Stowasser M, Bachmann AW, Huggard PR, Rossetti TR, Gordon RD . Treatment of familial hyperaldosteronism type I: only partial suppression of adrenocorticotropin required to correct hypertension. J Clin Endocrinol Metab 2000; 85: 3313–3318.
Gordon RD, Stowasser M, Tunny TJ, Klemm SA, Finn WL, Krek AL . Clinical and pathological diversity of primary aldosteronism, including a new familial variety. Clin Exp Pharmacol Physiol 1991; 18: 283–286.
Sukor N, Mulatero P, Gordon RD, So A, Duffy DL, Bertello C et al. Further evidence for linkage of familial hyperaldosteronism type II at chromosome 7p22 in Italian as well as Australian and South American families. J Hypertens 2008; 26: 1577–1582.
Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 2011; 331: 768–772.
Scholl UI, Goh G, Stölting G, de Oliveira RC, Choi M, Overton JD et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet 2013; 45: 1050–1054.
Geller DS, Zhang J, Wisgerhof MV, Shackleton C, Kashgarian M, Lifton RP . A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab 2008; 93: 3117–3123.
Therien B, Mellinger RC, Caldwell JR, Howard PJ . Primary aldosteronism due to adrenal hyperplasia. AMA Am J Dis Child 1958; 98: 90–99.
Gomez-Sanchez CE, Qi X, Gomez-Sanchez EP, Sasano H, Bohlen MO, Wisgerhof M . Disordered zonal and cellular CYP11B2 enzyme expression in familial hyperaldosteronism type 3. Mol Cell Endocrinol 2017; 439: 74–80.
Corey S, Clapham DE . Identification of native atrial G-protein-regulated inwardly rectifying K+ (GIRK4) channel homomultimers. J Biol Chem 1998; 273: 27499–27504.
Marionneau C, Couette B, Liu J, Li H, Mangoni ME, Nargeot J et al. Specific pattern of ionic channel gene expression associated with pacemaker activity in the mouse heart. J Physiol 2005; 562: 223–234.
Mintert E, Bösche LI, Rinne A, Timpert M, Kienitz M-C, Pott L et al. Generation of a constitutive Na+-dependent inward-rectifier current in rat adult atrial myocytes by overexpression of Kir3.4. J Physiol 2007; 585: 3–13.
Monticone S, Hattangady NG, Nishimoto K, Mantero F, Rubin B, Cicala MV et al. Effect of KCNJ5 mutations on gene expression in aldosterone-producing adenomas and adrenocortical cells. J Clin Endocrinol Metab 2012; 97: E1567–E1572.
Oki K, Plonczynski MW, Lam ML, Gomez-Sanchez EP, Gomez-Sanchez CE . The potassium channel, Kir3.4 participates in angiotensin II-stimulated aldosterone production by a human adrenocortical cell line. Endocrinology 2012; 153: 4328–4335.
Monticone S, Bandulik S, Stindl J, Zilbermint M, Dedov I, Mulatero P et al. A case of severe hyperaldosteronism caused by a de novo mutation affecting a critical salt bridge Kir3.4 residue. J Clin Endocrinol Metab 2015; 100: E114–E118.
Monticone S, Hattangady NG, Penton D, Isales CM, Edwards MA, Williams TA et al. A novel Y152C KCNJ5 mutation responsible for familial hyperaldosteronism type III. J Clin Endocrinol Metab 2013; 98: E1861–E1865.
Scholl UI, Nelson-Williams C, Yue P, Grekin R, Wyatt RJ, Dillon MJ et al. Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. Proc Natl Acad Sci USA 2012; 109: 2533–2538.
Mulatero P, Tauber P, Zennaro MC, Monticone S, Lang K, Beuschlein F et al. KCNJ5 mutations in European families with nonglucocorticoid remediable familial hyperaldosteronism. Hypertension 2012; 59: 235–240.
Tauber P, Penton D, Stindl J, Humberg E, Tegtmeier I, Sterner C et al. Pharmacology and pathophysiology of mutated KCNJ5 found in adrenal aldosterone-producing adenomas. Endocrinology 2014; 155: 1353–1362.
Murthy M, Xu S, Massimo G, Wolley M, Gordon RD, Stowasser M et al. Role for germline mutations and a rare coding single nucleotide polymorphism within the KCNJ5 potassium channel in a large cohort of sporadic cases of primary aldosteronism. Hypertension 2014; 63: 783–789.
Sertedaki A, Markou A, Vlachakis D, Kossida S, Campanac E, Hoffman DA et al. Functional characterization of two novel germline mutations of the KCNJ5 gene in hypertensive patients without primary aldosteronism but with ACTH-dependent aldosterone hypersecretion. Clin Endocrinol 2016; 85: 845–851.
Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2016; 101: 1889–1916.
Mulatero P, Monticone S, Rainey WE, Veglio F, Williams TA . Role of KCNJ5 in familial and sporadic primary aldosteronism. Nat Rev Endocrinol 2013; 9: 104–112.
Mussa A, Camilla R, Monticone S, Porta F, Tessaris D, Verna F et al. Polyuric-polydipsic syndrome in a pediatric case of non-glucocorticoid remediable familial hyperaldosteronism. Endocr J 2012; 59: 497–502.
Mulatero P, Di Cella SM, Monticone S, Schiavone D, Manzo M, Mengozzi G et al. 18-hydroxycorticosterone, 18-hydroxycortisol, and 18-oxocortisol in the diagnosis of primary aldosteronism and its subtypes. J Clin Endocrinol Metab 2012; 97: 881–889.
Adachi M, Muroya K, Asakura Y, Sugiyama K, Homma K, Hasegawa T . Discordant genotype-phenotype correlation in familial hyperaldosteronism type III with KCNJ5 gene mutation: a patient report and review of the literature. Horm Res Paediatr 2014; 82: 138–142.
Charmandari E, Sertedaki A, Kino T, Merakou C, Hoffman DA, Hatch MM et al. A novel point mutation in the KCNJ5 gene causing primary hyperaldosteronism and early-onset autosomal dominant hypertension. J Clin Endocrinol Metab 2012; 97: E1532–E1539.
Tong A, Liu G, Wang F, Jiang J, Yan Z, Zhang D et al. A novel phenotype of familial hyperaldosteronism type III: concurrence of aldosteronism and Cushing’s syndrome. J Clin Endocrinol Metab 2016; 101: 4290–4297.
Acknowledgements
PM and SM are in receipt of a grant from the Italian Ministry of the Instruction, University and Research (ex-60% 2013 and 2014, PM; ex-60% 2014 and 2015, SM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Monticone, S., Tetti, M., Burrello, J. et al. Familial hyperaldosteronism type III. J Hum Hypertens 31, 776–781 (2017). https://doi.org/10.1038/jhh.2017.34
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhh.2017.34
This article is cited by
-
Familial hyperaldosteronism type III a novel case and review of literature
Reviews in Endocrine and Metabolic Disorders (2019)