Abstract
Herein we report on three siblings with Leigh syndrome (LS) harboring a homoplasmic m.3697G>A mutation (G131S) in the MT-ND1 gene. The siblings’ phenotypically normal mother had the same, albeit heteroplasmic, mutation. Complex I deficiency (8% of average control values) was demonstrated in a biceps brachii muscle from one of the patients. Heteroplasmic m.3697G>A has been reported in patients with Leber’s hereditary optic neuropathy, mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes and Stüve–Wiedemann syndrome. Because all three patients in this series carried m.3697G>A in a homoplasmic manner and had LS, we suggest that homoplasmy of m.3697G>A may cause the LS phenotype.
Similar content being viewed by others
Introduction
Leigh syndrome (LS) is a subacute necrotizing encephalomyelopathy characterized by bilateral symmetric necrotic lesions of gray matter nuclei in the basal ganglia, diencephalon, cerebellum, or brainstem.1 Mutations in both nuclear- and mitochondrial-encoded genes involved in energy metabolism cause LS,1 with an increasing number of new mutations reported in recent years. The heteroplasmic mitochondrial mutation m.3697G>A has been identified in patients with Leber’s hereditary optic neuropathy, mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes and Stüve–Wiedemann syndrome,2, 3, 4, 5 indicating the pathogenicity of the mutation in mitochondrial disorders. Herein we report on three siblings with LS who harbored a homoplasmic m.3697G>A mutation, extending the significance of this mutation to the pathogenesis of LS.
Case reports
The present study was approved by the Institutional Review Board of the National Center of Neurology and Psychiatry, and the parents of the three children provided written informed consent.
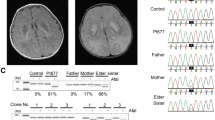
The three siblings in the present study were born from healthy parents and had no other siblings. Patient 1, a 9-year-old girl, presented with progressive gait disturbance from 1 year and 6 months of age. Brain magnetic resonance imaging at 2 years of age showed high signal intensity areas in the bilateral putamen and caudate nucleus on T2-weighted images (Figure 1a). Patient 1 was given a hearing aid when she was 3 years old. At the time of writing, Patient 1 exhibited dystonia of the upper and lower limbs but was able to walk slowly with support and was able to speak in sentences.
Magnetic resonance imaging findings in (a) Patient 1, (b) Patient 2 and (c) Patient 3. Axial T2-weighted images (TR 4600, TE 100) show high signal intensity areas in the bilateral putamen and caudate nucleus of Patient 1 (a), bilateral putamen, left globus pallidus, caudate nucleus and thalamus of Patient 2 (b), and bilateral putamen, caudate nucleus, globus pallidus and brainstem of Patient 3 (c).
Patient 2, a 7-year-old boy, started walking at 1 year of age; however, rigidity of the upper and lower limbs became prominent at 2 years of age. Brain magnetic resonance imaging revealed high signal intensity areas in the bilateral putamen, left globus pallidus, caudate nucleus and thalamus on T2-weighted images (Figure 1b). Currently, Patient 2 is showing progression of dystonia and cannot stand without support or speak in sentences.
Patient 3, a 5-year-old girl, started walking at 1 year and 3 months of age; however, she lost the ability to walk at 1 year and 6 months of age. Brain magnetic resonance imaging revealed high signal intensity areas in the bilateral putamen, caudate nucleus, globus pallidus and brainstem on T2-weighted images (Figure 1c). At 2 years of age, Patient 3 suddenly developed respiratory failure and muscle spasticity of the limbs and was started on artificial respiration. A tracheostomy and gastrostomy were performed, and Patient 3 is currently bedridden and being managed with artificial respiration at home. In all three siblings, the lactic acid concentration in the cerebrospinal fluid and blood was not elevated compared with the normal range (Supplementary Table).
Biochemical studies
The enzymatic activity of individual respiratory chain complexes was measured in a biceps brachii muscle specimen from Patient 1 and equivalent specimens from normal controls (n=5) according to the methods of Trounce et al.6 The activity of Complexes I–V is expressed relative to that of citrate synthase.7 Only the activity of Complex I was significantly reduced compared with control values (8% of average control values; Table 1).
Mitochondrial DNA (mtDNA) analyses
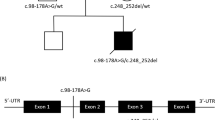
We carried out entire mtDNA sequencing after PCR amplification (primer sequences are available on request) as described elsewhere.8 Amplified fragment were directly sequenced using a BigDye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems, Foster City, CA, USA) and were run on an ABI 3700 automated sequencer (Applied Biosystems). Entire mtDNA sequencing of a biceps brachii muscle specimen from Patient 1 revealed that this patient harbored a homoplasmic m.3697G>A substitution (G131S). Subsequently, this mutation was confirmed using blood samples from all three siblings and their parents. All three siblings had a homoplasmic m.3697G>A mutation, whereas their mother had a heteroplasmic m.3697G>A mutation; the mutation was not present in their father (Figure 2a).
Discussion
The m.3697G>A mutation is located within the protein-coding region of the gene encoding ND1, a subunit of electron transport chain enzyme Complex I.2 Complex I is the first and largest enzyme of the respiratory chain, coupling electron transfer between NADH and ubiquinone to the translocation of four protons across the membrane.9 Complex I consists of 44 subunits.10 Many mutations in the subunits of Complex I have been associated with human neurodegenerative diseases.11, 12 The m.3697G>A mutation changes the 131st glycine into serine. This glycine is a highly conserved residue (Figure 2b) and is located in the loop between transmembrane domains C and D.2
Heteroplasmic m.3697G>A mutations have been reported in patients with Leber’s hereditary optic neuropathy, mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes and Stüve–Wiedemann syndrome2, 3, 4, 5 (Supplementary Table). The m.3697G>A mutation has been reported previously in a patient with LS, but detailed clinical information or the proportion of mutant mtDNA was not available.13 Because all three patients in our series carried homoplasmic m.3697G>A mutations and had LS, it is suggested that homoplasmy of m.3697G>A may cause the LS phenotype. Heteroplasmy of m.3697G>A may cause various mitochondrial disorders that presumably depend on the level of heteroplasmy. A correlation between mutation load and disease severity has been clearly demonstrated for the m.8993T>G and m.8993T>C mutations, in which case homoplasmy or a high mutation load causes LS, whereas lower mutation loads result in a less severe phenotype.14 Three other mutations in the gene encoding the ND1 subunit have been reported to be associated with LS, but these mutations are present in a heteroplasmic fashion.13 Therefore, of the MT-ND1 gene mutations identified to date, m.3697G>A is unique in causing LS only with an extremely high mutation load.
In mitochondrial disorders, lactic acid concentrations in the cerebrospinal fluid and blood are often increased;15 in the present study, lactic acid concentrations in the cerebrospinal fluid and blood were only marginally increased in all three siblings. However, this is similar to findings in other patients with mitochondrial disease due to m.3697G>A (Supplementary Table).
The mechanism whereby the three siblings developed homoplasmy, whereas their mother carried the mutation in a heteroplasmic manner, could be explained by the bottleneck theory.16 Amounts of mtDNA significantly decrease in early development of oocytes, and thus random selection of the mutated mtDNA could result in extensive skewing. Investigation of the ovary tissue could uncover the theory, but it is not possible to prove this theory in the pedigree in the present study because of lack of access to the ovarian tissue from the mother. Nonetheless, the bottleneck theory should be taken into account in the case of genetic counseling for mitochondrial disorders.
References
Rahman, S., Blok, R. B., Dahl, H. H., Danks, D. M., Kirby, D. M., Chow, C. W. et al. Leigh syndrome: clinical features and biochemical and DNA abnormalities. Ann. Neurol. 39, 343–351 (1996).
Kirby, D. M., McFarland, R., Ohtake, A., Dunning, C., Ryan, M. T., Wilson, C. et al. Mutations of the mitochondrial ND1 gene as a cause of MELAS. J. Med. Genet. 41, 784–789 (2004).
Spruijt, L., Smeets, H. J., Hendrickx, A., Bettink-Remeijer, M. W., Maat-Kievit, A., Schoonderwoerd, K. C. et al. A MELAS-associated ND1 mutation causing leber hereditary optic neuropathy and spastic dystonia. Arch. Neurol. 64, 890–893 (2007).
Morava, E., Hamel, B., Hol, F., Rodenburg, R. & Smeitink, J. Mitochondrial dysfunction in Stuve-Wiedemann syndrome in a patient carrying an ND1 gene mutation. Am. J. Med. Genet. A 140, 2248–2250 (2006).
Brandon, M. C., Lott, M. T., Nguyen, K. C., Spolim, S., Navathe, S. B., Baldi, P. et al. MITOMAP: a human mitochondrial genome database—2004 update. Nucleic Acids Res. 33, D611–D613 (2005).
Trounce, I. A., Kim, Y. L., Jun, A. S. & Wallace, D. C. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol. 264, 484–509 (1996).
Mimaki, M., Hatakeyama, H., Komaki, H., Yokoyama, M., Arai, H., Kirino, Y. et al. Reversible infantile respiratory chain deficiency: a clinical and molecular study. Ann. Neurol. 68, 845–854 (2010).
Akanuma, J., Muraki, K., Komaki, H., Nonaka, I. & Goto, Y. Two pathogenic point mutations exist in the authentic mitochondrial genome, not in the nuclear pseudogene. J. Hum. Genet. 45, 337–341 (2000).
Efremov, R. G. & Sazanov, L. A. Structure of the membrane domain of respiratory complex I. Nature 476, 414–420 (2011).
Balsa, E., Marco, R., Perales-Clemente, E., Szklarczyk, R., Calvo, E., Landazuri, M. O. et al. NDUFA4 is a subunit of complex IV of the mammalian electron transport chain. Cell Metab. 16, 378–386 (2012).
Sazanov, L. A. Respiratory complex I: mechanistic and structural insights provided by the crystal structure of the hydrophilic domain. Biochemistry 46, 2275–2288 (2007).
Schapira, A. H. Human complex I defects in neurodegenerative diseases. Biochim. Biophys. Acta 1364, 261–270 (1998).
Valente, L., Piga, D., Lamantea, E., Carrara, F., Uziel, G., Cudia, P. et al. Identification of novel mutations in five patients with mitochondrial encephalomyopathy. Biochim. Biophys. Acta 1787, 491–501 (2009).
White, S. L., Collins, V. R., Wolfe, R., Cleary, M. A., Shanske, S., DiMauro, S. et al. Genetic counseling and prenatal diagnosis for the mitochondrial DNA mutations at nucleotide 8993. Am. J. Hum. Genet. 65, 474–482 (1999).
Krageloh-Mann, I., Grodd, W., Schoning, M., Marquard, K., Nagele, T. & Ruitenbeek, W. Proton spectroscopy in five patients with Leigh's disease and mitochondrial enzyme deficiency. Dev. Med. Child Neurol. 35, 769–776 (1993).
Carling, P. J., Cree, L. M. & Chinnery, P. F. The implications of mitochondrial DNA copy number regulation during embryogenesis. Mitochondrion 11, 686–692 (2011).
Acknowledgements
This work was supported by Grants-in-Aid of the Research on Intractable Diseases (Mitochondrial Disorder) from the Ministry of Health, Labour and Welfare of Japan and the Intramural Research Grant (24–8) for Neurological and Psychiatric Disorders of NCNP (to YG).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Negishi, Y., Hattori, A., Takeshita, E. et al. Homoplasmy of a mitochondrial 3697G>A mutation causes Leigh syndrome. J Hum Genet 59, 405–407 (2014). https://doi.org/10.1038/jhg.2014.41
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2014.41
This article is cited by
-
The Korean undiagnosed diseases program phase I: expansion of the nationwide network and the development of long-term infrastructure
Orphanet Journal of Rare Diseases (2022)
-
Preimplantation genetic diagnosis for a carrier with m.3697G > A mitochondrial DNA mutation
Journal of Assisted Reproduction and Genetics (2021)