Abstract
Hearing impairment (HI) is the decreased ability to hear and discriminate among sounds. It is one of the most common birth defects. Epidemiological data show that more than one child in 1000 is born with HI, whereas more than 50% of prelingual HI cases are found to be hereditary. So far, 95 published autosomal-recessive nonsyndromic HI (ARNSHI) loci have been mapped, and 41 ARNSHI genes have been identified. In this study, we performed a genome-wide linkage study in a consanguineous Tunisian family, and report the mapping of a novel ARNSHI locus DFNB80 to chromosome 2p16.1-p21 between the two single-nucleotide polymorphisms rs10191091 and rs2193485 with a maximum multipoint logarithm of odds score of 4.1. The screening of seven candidate genes, failed to reveal any disease-causing mutations.
Similar content being viewed by others
Introduction
Hearing impairment (HI) is the most common sensorineural disorder in humans, and congenital HI affects about 0.16% of the newborns.1, 2 Approximately half of these cases are estimated to be genetically determined, and the remaining cases are likely to have an environmental cause.3 Approximately 80% of hereditary nonsyndromic HI is autosomal-recessive nonsyndromic HI (ARNSHI). Up to now, more than 95 chromosomal loci (known as DFNB) have been localized for ARNSHI, and the responsible genes have been identified in about 50% of DFNB loci (http://hereditaryhearingloss.org/). This heterogeneity is largely due to the complexity of the inner ear, and the various mechanisms that can lead to the HI phenotype.4
During the 1990s, microsatellite genome-wide linkage study in large families was used to map novel HI loci.5, 6, 7 Since the first years of this century, higher throughput and cost-effective single-nucleotide polymorphism genome scans have been increasingly used in place of it.8 Identification of corresponding HI-causing genes was based on functional candidate gene strategy and Sanger sequencing (pejv myo). Recently, high-throughput next-generation exome and target-region sequencing is changing the face of gene discovery (exome target). Although in the past, it would take on average 1–5 years to identify a novel gene leading to HI, today, the genetic basis for HI can be determined within months.
In the present study, we describe the identification in a Tunisian consanguineous family of a new ARNSHI locus named DFNB80, which maps to the chr2p16.1-p21 region.
Materials and methods
Family data
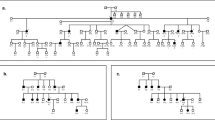
In this study, we investigated a seven-generation Tunisian family transmitting an autosomal-recessive HI (Figure 1). Informed consent was obtained from all participants and parents of subjects younger than 18 years of age. The pedigree structure is based upon interviews with multiple family members. Clinical history interviews and physical examinations of members of this family ruled out the implication of environmental factors for causing the HI. All affected individuals have a history of prelingual hearing loss (HL) and use sign language for communication. Pure-tone audiometry tests were performed to test air and bone conductions in all members. Tympanometry and caloric tests were also performed.
Pedigree and chromosome 2 SNPs that cosegregate with ARNSHI in Tunisian family. Black symbols represent hearing-impaired individuals. Clear symbols represent unaffected individuals. The haplotypes of the affected individuals define rs10191091 SNP as the proximal flanking; rs847808 SNP defined the distal flanking. The haplotype of the unaffected individual VI:2 defined a novel proximal flanking with rs2193485 SNP. The linked haplotypes are boxed.
Extraction of Genomic DNA
Venous blood samples were collected from a total of 11 individuals, including 5 who are hearing impaired. Genomic DNA was extracted from whole blood following a standard protocol9 and diluted to 50 ng μl−1.
Genome-wide analysis
The quality of the genomic DNA was assessed by spectrophotometric analysis and gel electrophoresis. Each of the 11 DNA family members was genotyped using the HumanCyto12v2.0 beadchip (Illumina Inc, San Diego, CA, USA). This chip used the Infinium II Assay to interrogate over 300 000 single-nucleotide polymorphisms (SNPs) and additional markers, targeting all regions of known cytogenetic importance on a single BeadChip. Each microarray was scanned using the iScan Array Scanner with high-resolution scanning upgrade (Illumina); files were analyzed using GenomeStudio Genotyping Module (Illumina).
Statistical analysis
Multipoint nonparametric linkage analysis was performed using Merlin v1.0.1 (http://www.sph.umich.edu/csg/abecasis/Merlin) and Simwalk 2.91 using easylinkage v5.08.10 The disease allele frequency was set at 0.01. In addition, homozygosity mapping was performed using plink v1.07.11 Copy number variation (CNV) analysis was performed by importing fluorescent signals into the BeadStudio software version 3.3 (Illumina). Intensities were normalized against a reference panel of 120 HapMap samples. QuantiSNP, PennCNV and VanillaICE CNV-detection algorithms were used to detect alterations using an online webtool for CNV analysis.12
Candidate gene screening
The potential candidate genes in the linked interval region on chromosome 2 were identified using the UCSC Genome Build 37.1 (University of California, Santa Cruz, http://genome.ucsc.edu/) and Endeavor Web-based software (http://homes.esat.kuleuven.be/~bioiuser/endeavour/index.php).13 A set of criteria including gene functions, gene-expression patterns, protein interactions and literature review was used for candidate gene prioritization (Supplementary Table 1). The primers used for the amplification and subsequent sequencing of exons and exon–intron boundaries of the candidate genes namely SPTBN1 [NM_003128], RTN4 [NM_207520.1], RHOQ [NM_012249], CRIPT [NM_014171], KCNK12 [NM_022055], CALM2 [NM_001743] and ATP6V1E1 [NM_001696] were designed by using Primer3 web software (http://frodo.wi.mit.edu/).14 PCR products were sequenced using an ABI 3100-Avant automated DNA sequencer and Big Dye Terminator Sequencing Kit (Applied Biosystems, California, CA, USA). Two hearing-impaired subjects, VII:1 and VII:3 (Figure 1), were investigated for the presence of mutations.
Results
Clinical data
All affected individuals in our Tunisian family showed congenital HL. For five affected individuals, the audiometric tests showed bilateral profound HI involving all frequencies (Figure 2). The HL did not appear to be progressive, with no correlation between severity of HL and current age. Vestibular function appeared to be normal in affected individuals. The hearing-impaired family members underwent a clinical examination for defects in ear morphology, dysmorphic facial features, eye disorders, including night blindness, and other clinical features that could indicate that the HL was syndromic. Clinical evaluation suggested no skin or renal anomalies. Funduscopic examinations of affected individuals showed no signs of retinitis pigmentosa. We therefore concluded that no other disorder was cosegregating with HL in this family.
Genome-wide analysis
Results of the genome-wide SNP genotyping analysis showed significant evidence of linkage to chromosome 2 (chr2p14-p21). The maximum multipoint logarithm of odds score is 4.1 between rs6728881 and rs7577004. This homozygous region was observed only in the five affected family members (Figure 1). Based on the affected individuals, the region is located on chr2p14-p21 between rs10191091 (chr2:46,573,210) and rs847808 (chr2:64,994,005). This region is 18.4 Mb and contains 145 genes (NCBI build 37.1). However, when based on individual VI:2 (nonaffected), the region can be narrowed down to 9.9 Mb (chr2p16.1-p21) between the two SNPs: rs10191091 (chr2:46,573,210) and rs2193485 (chr2:56,492,112). The homozygous region in individual VI:2 compromises about 8.5 Mb and 1202 genotyped SNPs (of which 355 are polymorph in the family) with the same genotype as the linked region. Microarray analysis revealed no CNVs in this region. No functional sequence variants were seen in the exon and exon–intron boundaries of seven candidate genes (Supplementary Table 1).
Discussion
In this paper, we report a new locus for autosomal-recessive HL mapped to chr2p16.1-p21. There are two known deafness genes on chromosome 2: OTOF (NM_194248) and PJVK (NM_001042702) that were identified from the DFNB915 and DFNB5916 loci, respectively, and six known loci, namely DFNB27,5 DFNB47,6 DFNA16,17 DFNA43,18 DFNA58 (Lezirovitz et al.7) and DFNA60, for which the causative gene has not yet been identified (Figure 3).
The novel locus on chromosome 2 (chr2p16.1-p21) overlaps with DFNA58 locus (chr2p12-p21), for which the gene has not yet been identified.7 It should be noted that there are several examples in which a recessive and a dominant form of deafness result from abnormalities of the same gene. For example, the GJB2 gene (gap junction protein, beta-2) locates on chromosome 13q12 and encodes the connexin 26, a transmembrane protein involved in cell–cell attachment of almost all tissues. GJB2 mutations cause autosomal-recessive (DFNB1),19 and sometimes dominant (DFNA3),20 nonsyndromic sensorineural HI. The DFNA3 mutations are likely to have dominant-negative effects with the formation of a channel that is not functional.21 Another example is the MYO7A gene in which several mutations are responsible for the recessive form of deafness. These mutations are distributed throughout the gene with the exception of the ‘coiled-coil’ region responsible for dimerization of myosin 7A. A deletion of 9 bp located in this region is responsible for an autosomal-dominant deafness (DFNA11).22
In the candidate region of 9.9 Mb, we have sequenced the coding region of seven candidate genes (Supplementary Table 1), and we have not been able to identify the disease-causing gene. Because only the exonic and promoter regions were sequenced, the possibility of a functional variant in the intronic regions cannot be ruled out. Next-generation sequencing might identify the disease-causing mutation in this region.
References
Mehl, A. L. & Thomson, V. Newborn hearing screening: the great omission. Pediatrics 101, E4 (1998).
Mehl, A. L. & Thomson, V. The Colorado newborn hearing screening project, 1992-1999: on the threshold of effective population-based universal newborn hearing screening. Pediatrics 109, E7 (2002).
Roizen, N. J. Nongenetic causes of hearing loss. Ment. Retard. Dev. Disabil. Res. Rev. 9, 120–127 (2003).
Steel, K. P. & Bussoli, T. J. Deafness genes: expressions of surprise. Trends Genet. 15, 207–211 (1999).
Pulleyn, L. J., Jackson, A. P., Roberts, E., Carridice, A., Muxworthy, C., Houseman, M. et al. A new locus for autosomal recessive non-syndromal sensorineural hearing impairment (DFNB27) on chromosome 2q23-q31. Eur. J. Hum. Genet. 8, 991–993 (2000).
Hassan, M. J., Santos, R. L., Rafiq, M. A., Chahrour, M. H., Pham, T. L., Wajid, M. et al. A novel autosomal recessive non-syndromic hearing impairment locus (DFNB47) maps to chromosome 2p25.1-p24.3. Hum. Genet. 118, 605–610 (2006).
Lezirovitz, K., Braga, M. C., Thiele-Aguiar, R. S., Auricchio, M. T., Pearson, P. L., Otto, P. A. et al. A novel autosomal dominant deafness locus (DFNA58) maps to 2p12-p21. Clin. Genet. 75, 490–493 (2009).
Arnett, J., Emery, S. B., Kim, T. B., Boerst, A. K., Lee, K., Leal, S. M. et al. Autosomal dominant progressive sensorineural hearing loss due to a novel mutation in the KCNQ4 gene. Arch. Otolaryngol. Head Neck Surg. 137, 54–59 (2011).
Grimberg, J., Nawoschik, S., Belluscio, L., McKee, R., Turck, A., Eisenberg, A. et al. A simple and efficient non-organic procedure for the isolation of genomic DNA from blood. Nucleic Acids Res. 17, 8390 (1989).
Hoffmann, K. & Lindner, T. H. easyLINKAGE-Plus—automated linkage analyses using large-scale SNP data. Bioinformatics 21, 3565–3567 (2005).
Purcell, S., Neale, B., Todd-Brown, K., Thomas, L., Ferreira, M. A., Bender, D. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Vandeweyer, G., Reyniers, E., Wuyts, W., Rooms, L. & Kooy, R. F. CNV-webstore: online CNV analysis, storage and interpretation. BMC Bioinformatics 12, 4 (2011).
Tranchevent, L. C., Barriot, R., Yu, S., Van Vooren, S., Van Loo, P., Coessens, B. et al. ENDEAVOUR update: a web resource for gene prioritization in multiple species. Nucleic Acids Res. 36, W377–W384 (2008).
Rozen, S. & Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132, 365–386 (2000).
Yasunaga, S., Grati, M., Cohen-Salmon, M., El-Amraoui, A., Mustapha, M., Salem, N. et al. A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. Nat. Genet. 21, 363–369 (1999).
Delmaghani, S., del Castillo, F. J., Michel, V., Leibovici, M., Aghaie, A., Ron, U. et al. Mutations in the gene encoding pejvakin, a newly identified protein of the afferent auditory pathway, cause DFNB59 auditory neuropathy. Nat. Genet. 38, 770–778 (2006).
Fukushima, K., Kasai, N., Ueki, Y., Nishizaki, K., Sugata, K., Hirakawa, S. et al. A gene for fluctuating, progressive autosomal dominant nonsyndromic hearing loss, DFNA16, maps to chromosome 2q23-24.3. Am. J. Hum. Genet. 65, 141–150 (1999).
Flex, E., Mangino, M., Mazzoli, M., Martini, A., Migliosi, V., Colosimo, A. et al. Mapping of a new autosomal dominant non-syndromic hearing loss locus (DFNA43) to chromosome 2p12. J. Med. Genet. 40, 278–281 (2003).
Kelsell, D. P., Dunlop, J., Stevens, H. P., Lench, N. J., Liang, J. N., Parry, G. et al. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 387, 80–83 (1997).
Denoyelle, F., Lina-Granade, G., Plauchu, H., Bruzzone, R., Chaïb, H., Lévi-Acobas, F. et al. Connexin 26 gene linked to a dominant deafness. Nature 393, 319–320 (1998).
Dupont, E., el Aoumari, A., Briand, J. P., Fromaget, C. & Gros, D. Cross-linking of cardiac gap junction connexons by thiol/disulfide exchanges. J. Membr. Biol. 108, 247–252 (1989).
Liu, X. Z., Walsh, J., Mburu, P., Kendrick-Jones, J., Cope, M. J., Steel, K. P. et al. Mutations in the myosin VIIA gene cause non-syndromic recessive deafness. Nat. Genet. 16, 188–190 (1997).
Acknowledgements
We thank all the family members for their cooperation. This work was supported by Ministère de l’enseignement supérieur et de la Recherche Scientifique, Tunisia, University of Antwerp, Belgium, and the Research Foundation Flanders (FWO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Ali Mosrati, M., Schrauwen, I., Ben Saiid, M. et al. Genome-wide analysis reveals a novel autosomal-recessive hearing loss locus DFNB80 on chromosome 2p16.1-p21. J Hum Genet 58, 98–101 (2013). https://doi.org/10.1038/jhg.2012.141
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2012.141