Abstract
Using a combination of process rate determination, microsensor profiling and molecular techniques, we demonstrated that denitrification, and not anaerobic ammonium oxidation (anammox), is the major nitrogen loss process in biological soil crusts from Oman. Potential denitrification rates were 584±101 and 58±20 μmol N m−2 h−1 for cyanobacterial and lichen crust, respectively. Complete denitrification to N2 was further confirmed by an 15NO3− tracer experiment with intact crust pieces that proceeded at rates of 103±19 and 27±8 μmol N m−2 h−1 for cyanobacterial and lichen crust, respectively. Strikingly, N2O gas was emitted at very high potential rates of 387±143 and 31±6 μmol N m−2 h−1 from the cyanobacterial and lichen crust, respectively, with N2O accounting for 53–66% of the total emission of nitrogenous gases. Microsensor measurements revealed that N2O was produced in the anoxic layer and thus apparently originated from incomplete denitrification. Using quantitative PCR, denitrification genes were detected in both the crusts and were expressed either in comparable (nirS) or slightly higher (narG) numbers in the cyanobacterial crusts. Although 99% of the nirS sequences in the cyanobacterial crust were affiliated to an uncultured denitrifying bacterium, 94% of these sequences were most closely affiliated to Paracoccus denitrificans in the lichen crust. Sequences of nosZ gene formed a distinct cluster that did not branch with known denitrifying bacteria. Our results demonstrate that nitrogen loss via denitrification is a dominant process in crusts from Oman, which leads to N2O gas emission and potentially reduces desert soil fertility.
Similar content being viewed by others
Introduction
The availability of nitrogen is one of the major constraints to biological activities in arid ecosystems (Muyi and Caldwell, 1997; Garbauer and Ehleringer, 2000), and thus estimates of the inputs and outputs of nitrogen in deserts is essential to the understanding and future predictions of ecosystem functioning therein. Biological soil crusts (referred to as crusts hereafter), typically covering surface desert soils, greatly influence the nitrogen cycle of arid and semiarid ecosystems (Belnap, 2001). The conversion of atmospheric N2 into ammonium (NH4+) by crustal diazotrophic cyanobacteria, lichens and heterotrophic bacteria is the dominant source of nitrogen in desert ecosystems (Belnap, 2002; Billings et al., 2003; Yeager et al., 2004; Veluci et al., 2006; Abed et al., 2010), particularly where rainfall and anthropogenic nitrogen inputs are minimal. Although much progress in nitrogen cycling research in crusts has so far been made with respect to nitrogen inputs via nitrogen fixation, nitrogen loss processes have received much less attention. Nitrogen is lost as gaseous products (that is, N2, N2O and NO) through the stepwise reduction of nitrate by denitrification (NO3−→NO2−→NO→N2O→N2), the combination of ammonium and nitrite by anammox (NH4++NO2−→N2), nitrifier-denitrification by ammonia-oxidizers under oxygen stress or, to a lesser extent, as a by-product during aerobic ammonia oxidation (Ritchie and Nicholas, 1972; Poth, 1986; Wrage et al., 2001; Schmidt et al., 2004; McNeill and Unkovich, 2007; Santoro et al., 2011). Denitrification and anammox have been recognized as the major nitrogen loss mechanisms in many environments, including freshwater, marine as well as terrestrial ecosystems (Seitzinger, 1988; Peterjohn and Schlesinger, 1991; Kuypers et al., 2003, 2005; Ward et al., 2009; Humbert et al., 2010; Lam and Kuypers, 2011), but their potential occurrence and significance in crusts remains poorly understood. Although crusts have been shown to emit N2O and NO with amounts dependent on season and N2-fixation rates (Barger et al., 2005; Crenshaw et al., 2008), it was not clear which process was responsible for the production of these gases. Rate measurements were performed on crusts from Colorado Plateau, Mojave, Sonoran and Chihuahuan deserts in the United States, and all of them displayed extremely low rates of denitrification and anammox, while ammonia oxidation rates in these crusts were comparable with N2-fixation rates (Johnson et al., 2005, 2007; Strauss and Day, 2012). Subsequent nitrogen budget calculations suggested that these crusts act as a net nitrogen source. It is not known if such perceived imbalance is widespread among desert ecosystems in other parts of the globe, and this has prompted our current study.
With deserts covering approximately one-third of the Earth’s land surface (ca. 44 million km2), the subtropical Arabian Desert covers almost the entire Arabian Peninsula and is considered as the fourth largest desert (ca. 2.3 million km2) in the world (Allan and Warren, 1993). Nevertheless, crusts covering the surface soils of these deserts remain understudied. With respect to nitrogen cycle, crusts from Oman were recently shown to fix nitrogen at rates (39.4±13.4–58.5±2.6 μmol C2H2 m−2 h−1 as determined by acetylene reduction assay) comparable with those measured in other desert ecosystems (Abed et al., 2010). However, no information is available on other nitrogen transformation processes, despite their apparent paramount importance in regulating nitrogen balance within these systems. A primary unique characteristic of arid and semiarid desert ecosystems is the episodic nature of water availability, which has significant consequences for carbon and nitrogen cycling. Biological processes (such as respiration, photosynthesis and nitrogen fixation) become active within seconds to a few hours after wetting (Isichei, 1980; Belnap, 1995; Garcia-Pichel and Belnap, 1996; Gao et al., 1998; Qiu and Gao, 1999; Issa et al., 2001; Austin et al., 2004; Qiu et al., 2004). As a consequence of the high respiration activities, oxygen becomes limiting very rapidly, resulting in the formation of anoxic microsites (Garcia-Pichel and Belnap, 1996) and thereby conditions suitable for anaerobic processes. Indeed, a recent study demonstrated the occurrence of the strictly anaerobic methanogenesis process in crusts from the Negev Desert, even when incubated under natural oxic conditions (Angel et al., 2011). Similarly, such anoxic conditions would be conducive to denitrification and anammox, which we hypothesize to be the key nitrogen loss processes in crusts that become active after precipitation events, taking advantage of the developed anoxic conditions and the readily available organic matter produced by photosynthesis.
To test this hypothesis, we measured denitrification and anammox rates under completely anoxic conditions, as well as in intact crusts for denitrification only, in two types of crusts—cyanobacteria- and lichen-dominated—from the desert of Oman. These rates were determined by using the acetylene inhibition technique and 15N-labeling experiments. Microsensor measurements were also made to locate denitrification activity and the concomitant N2O emission in intact pieces of crusts with and without acetylene addition. Potential rates of ammonium oxidation were measured to reveal the contribution of nitrification to the nitrate pool in the crusts and to the production of N2O gas. A suite of key functional genes in nitrogen cycling were quantified at both gene abundance and expression levels by using quantitative PCR (qPCR) and reverse transcription qPCR (RT-qPCR), whereas the diversity of selected biomarker genes were further analyzed.
Materials and methods
Samples origin and site description
The topmost 1–2 cm of cyanobacteria- (free of symbiotic fungi) and lichen- (composite organisms consisting of a symbiotic relationship between a fungus and a photosynthetic partner, usually either cyanobacteria or microalgae)-dominated crusts, were collected from Wadi Al-Khoud, northern Oman (23°04.595′ N, 58°09.055′ E). The site is surrounded by low limestone hills and the surface is covered with small- to medium-sized gravels. Rainfall occurs in the area between 2 and 5 times a year, with a mean annual rainfall of ca. 86.2 mm from November to April. Temperature ranges between 47 and 50 °C in the summer and as low as 12 °C in the winter. Both cyanobacteria- and lichen-dominated crusts contained more sand (49–50.7%) than silt and clay (Table 1). The pH of the two crust types ranged between 7.25 and 7.45, indicating a slight alkalinity of the crusts, and the cyanobacterial crusts had a significantly lower electrical conductivity than the lichen crusts (Table 1). The samples were collected in May 2011, kept dry in plastic boxes and shipped to the Max-Planck Institute, Bremen, Germany where all incubations were performed within 3 months after collection. The last precipitation event before sampling was on 22 January 2011.
Determination of denitrification rates
Two types of rate measurements were made in order: (1) to assess the maximum potential of nitrogenous gas production by denitrification under ideal conditions (hereafter referred to as potential rates), and (2) to estimate the rate of complete denitrification to N2 in intact crusts.
Potential denitrification rates (that is, the maximum rates under fully anoxic conditions without limitation by nitrate availability and diffusive solute transport) were measured in slurry incubations of crusts by using the acetylene inhibition technique (Sørensen, 1978). The addition of acetylene inhibits the last step of denitrification (that is, the conversion of N2O to N2) and thus results in the accumulation of N2O. Crust samples (2–3 g each) were incubated in 110-ml glass bottles filled with 30 ml sterile anoxic distilled H2O (bubbled with helium gas for 15 min) amended with 25 μM NO3–, thus leaving a headspace volume of 80 ml. Each bottle was wrapped with an aluminum foil, to prevent O2 production by photosynthesis, and sealed gas-tight with a rubber stopper, through which it was purged with N2 gas for around 10 min. Also, 10% of the headspace volume was replaced by acetylene, and the bottles were shaken on a plate shaker at 150 r.p.m. Gas samples (1 ml each) were drawn from the headspace using a gas-tight syringe at different time intervals (that is, 5 min, 2, 4, 6 and 8 h) and injected into gas-tight sample vials (3 ml exetainers, Labco, Ceredigion, Wales, UK) pre-flushed with N2. N2O concentrations in the exetainers were then measured on a gas chromatograph equipped with a 63Ni electron-capture detector (GC 7890; Agilent Technologies, Boeblingen, Germany). The linear increase in N2O concentration observed between 4 and 8 h of incubation was used to calculate the denitrification rate per cm2 of crust. The weight of crusts was converted to area unit by using a factor that was obtained after weighing 20 crust pieces of 1 cm2 surface area each. An equivalent series of crust pieces was incubated without the addition of acetylene to quantify the potential rate of N2O emission. All incubations were run in six replicates. Additional incubations without nitrate and with autoclaved crust pieces served as negative controls.
Denitrification rates were determined in intact crust pieces with vertical oxygen concentration gradients maintained by photosynthesis and respiration and with solute transport governed by diffusion, thus more likely resembling natural conditions. Crust pieces (3–4 g each) were incubated with 25 μM 15NO3− in an experimental setup similar to the one described above with few modifications. At the beginning of the experiment, the headspace of the bottles was adjusted to 80% helium (He) and 20% oxygen (O2) to allow O2 gradients to develop in the crust pieces. The bottles were incubated at 26 °C, in the light without shaking. Gas samples (3 ml each) were drawn from the headspace at different time intervals and injected into 6-ml exetainers that were completely filled with He-saturated dH2O. After injection of the gas sample into the exetainer, 100 μl 50% zinc chloride (ZnCl2) was added to stop biological activity. All incubations were run in triplicates and autoclaved crust pieces served as negative controls. The excess concentrations of 29N2 and 30N2 in the headspace were derived from the 29N2:28N2 and 30N2:28N2 ratios determined by gas chromatography-isotope ratio mass spectrometry (GC-IRMS; VG Optima; Isotech, Merseyside, UK). Due to the unexpectedly high background concentrations of 14NO3− in the dry crusts (Table 1), the mole fraction of 15NO3− (F15) was as low as 20–26%. Thus, random isotope pairing in the denitrification process was expected to produce 5.7–8 times more 29N2 than 30N2 (Nielsen, 1992). Accordingly, the 29N2 time-series data were of much better quality than the 30N2 time-series data and consequently were used to calculate the production rate of 28N2+29N2+30N2 (RN2tot) from the production rate of 29N2 (R29N2) as follows (Nielsen, 1992):

This calculation method was justified because anammox rates were not detectable (see Results) and therefore 29N2 production exclusively originated from denitrification. R29N2 was derived from the linear increase in 29N2 concentration observed between 4 and 8 h of incubation, and RN2tot was then expressed per cm2 of crust. F15(NO3−) was determined from the size of the initial nitrate pool in the dry crust, which was measured after suspending an additional series of pre-weighed crust pieces in 3 ml MilliQ dH2O, immediately followed by centrifugation at 1500 g for 10 min and analysis of NOx− in the supernatant with the VCl3 reduction assay (Braman and Hendrix, 1989) on a NOx analyzer (CLD 86; Eco-Physics, Munich, Germany). Additionally, the intracellular NO3− pool present in the dry crusts was quantified by subjecting freshly wetted crust pieces to three freeze-thaw cycles (−180 °C vs +90 °C) to make vacuolated cells burst and release their cellular contents into the medium (Kamp et al., 2011).
Determination of anammox rates
To check for the occurrence of anaerobic ammonium oxidation (anammox), crust samples were incubated in 30 ml sterile MilliQ dH2O amended with 25 μM 15NH4Cl and 25 μM Na14NO2− in 110-ml glass bottles. The bottles were flushed with He for 10 min to create completely anoxic conditions, wrapped with aluminum foil to prevent O2 production by photosynthesis and incubated at 26 °C on a plate shaker (100 r.p.m.). Gas samples (2.5 ml each) from the headspace were collected at different time intervals and transferred into 6-ml exetainers filled completely with He-saturated water. After injection of the gas sample into the exetainer, 100 μl 50% ZnCl2 was added to stop any biological activity. The excess concentration of 29N2 in the headspace was determined from the 29N2:28N2 ratio by GC-IRMS. All incubations were run in triplicates and autoclaved crust pieces served as negative controls. To determine F15(NH4+), the size of the initial NH4+ pool in the dry crusts was measured in the same supernatants as used for background nitrate measurements as previously described (Kempers and Kok, 1989). F15(NH4+) was 65% and 64% in the cyanobacterial and lichen crust incubations, respectively.
Potential ammonia oxidation rates
Potential rates of ammonia oxidation, the first step in nitrification, in crusts were measured as previously described (Stief and de Beer, 2006), to allow budget calculations of N input and output processes. Briefly, pre-weighed crust pieces (2–3 g each) were slurried in 30 ml sterile MilliQ dH2O in 110-ml glass bottles. For the measurement of ammonia oxidation, the medium contained 25 μM ammonium sulfate ((NH4)2SO4) and 20 mM sodium chlorate (NaClO3) that inhibits nitrite oxidation (that is, the second step of nitrification (Belser and Mays, 1980)). The rate of ammonium oxidation can then be inferred from the linear increase in nitrite (NO2−) concentration during the incubation. This assumes that NH4+ is quantitatively converted to NO2− rather than N2O, which was justified here because our microsensor measurements confirmed the absence N2O production in the oxic layer of the crusts. All bottles were placed on a multiple stirrer and stirred gently at 100 r.p.m. throughout the incubation. To ensure non-limiting O2 availability, the medium was purged with air using a glass pipette connected to an air pump. Subsamples of the slurry (2 ml each) were collected after 5 min, 2, 4, 6 and 8 h and were immediately frozen at −20 °C until analyzed. At the end of the experiment, all samples were thawed and centrifuged at 5000 r.p.m. for 10 min to remove soil particles. In the particle-free water samples, NO2− was analyzed using the sodium iodide (NaI) reduction assay (Yang et al., 1997) on a NOx analyzer (CLD 86; Eco-Physics). All incubations were run in triplicates at 26 °C, and autoclaved crust pieces served as negative controls.
Microsensor measurements
Crust pieces were immobilized in petri dishes using modeling clay and then wetted by the addition of sterile MilliQ dH2O. Oxygen and N2O microprofiling was started immediately after wetting. Oxygen profiles were measured in the light and in the dark, while N2O profiles were measured in the light only. The profiles were measured at 100 μm steps using Clark-type sensors with an internal reference and a guard cathode (Revsbech, 1989; Andersen et al., 2001). The O2 sensor was calibrated at a temperature of 26 °C by taking the readings in the anoxic layer of a piece of crust and in the fully aerated MilliQ dH2O (Revsbech, 1989). The N2O sensor was calibrated by taking several readings after the addition of aliquots of an N2O-saturated stock solution to 100 ml MilliQ dH2O, thereby covering a concentration range of 0–112 μM N2O. Microprofiles were measured using an automated setup controlled by a computer (Polerecky et al., 2007). Microsensor positioning with 1 μm precision was facilitated by a VT-80 linear positioner (Micros, Eschbach, Germany) equipped with a DC motor (Faulhaber, Schoenaich, Germany). The microsensors were connected to fast-responding picoamperemeters and the signals were collected using a data acquisition device (DAQPAD-6015, National Instruments, Munich, Germany). Areal rates or fluxes of O2 and N2O production were calculated from the linear concentration gradients that developed in the crust pieces using the Fick’s law of diffusion. The diffusion coefficients of O2 (2.49 × 10−9 m2 s−1) and N2O (2.40 × 10−9 m2 s−1) at 26 °C in water were converted to effective diffusivities in the crusts using a porosity value of 0.6 according to Ullman and Aller (1982).
qPCR and expression of functional genes
Cyanobacterial and lichen crust samples were subjected to DNA extraction using the PowerBiofilm DNA isolation kit (MOBIO laboratories, Inc., Carlsbad, CA, USA) according to the manufacturer’s instructions. qPCR was performed to target key functional genes in the first two steps in denitrification: nitrate reduction (narG and napA encoding membrane-bound and periplasmic nitrate reductases, respectively) and nitrite reduction (nirS encoding cytochrome cd1-containting nitrite reductase); as well as that for both archaeal and bacterial ammonia oxidation (amoA encoding the ammonia monooxygenase subunit A) using previously described primers (Table 3) and protocols (Lam et al., 2009). All qPCR reactions were run on an iCycler iQ Real-Time PCR Detection System equipped with iQ5 software (Bio-Rad, Munich, Germany) and with the PowerSYBR Green PCR Master Mix (Applied Biosystems, Darmstadt, Germany). The copy number of the genes was calculated after correcting for the concentration and dilution factors and are presented as copy number per g of crust (Table 3).
The expression of narG and nirS was additionally followed in both the crust types after 5 min, 2, 4, 6 and 8 h of wetting with sterile water. The wet pieces were incubated in plastic petri dishes at 26 °C under light. The mRNA was first extracted from the crust samples using the PowerBiofilm RNA isolation kit (MOBIO laboratories, Inc.,) according to the manufacturer’s instructions. RNA was reverse-transcribed using Superscript III First-Strand Synthesis Master Mix (Invitrogen, Darmstadt, Germany) with narG and nirS-specific antisense primers. The cDNA was then treated with RNaseH (Ambion, Darmstadt, Germany, Applied Biosystems) at 37 °C for 20 min, immediately kept on ice for 1 min and then diluted 2.5 times with nuclease-free water. Subsequent qPCRs were performed with the obtained cDNA as described above. DNA contamination in RNA extracts were also checked and ruled out by performing parallel qPCRs directly with the RNA extracts.
Pyrosequencing of nirS and nosZ
Purified DNA extracts from both the cyanobacterial and lichen crusts were submitted to the Research and Testing Laboratory (RTL; Lubbock, TX, USA) for tag-pyrosequencing of amplified gene fragments of nirS, along with nosZ (nitrous oxide reductase encoding gene) which is a biomarker gene for the last step of denitrification. Tag-encoded FLX amplicon pyrosequencing (bTEFAP) was performed as described before (Dowd et al., 2008a, 2008b) using the GS FLX titanium sequencing kit XLR70, Roche Applied Science (Indianapolis, IN, USA). One-step PCR was conducted with a mixture of hot-start and hot-start high-fidelity Taq polymerases along with primers specific for either nirS or nosZ. The bTEFAP sequencing was performed according to the RTL protocols (www.researchandtesting.com). Obtained sequences were trimmed, low-quality ends and tags were removed and were checked for chimeras using custom software (Dowd et al., 2008b) and the Black Box Chimera Check software B2C2 (http://www.researchandtesting.com/B2C2.html). Sequences shorter than 250 bp were excluded from further analysis. The program MUSCLE was used for unstructured multiple sequence alignment using non-default parameters –maxiters 2, -diags, which were shown to minimize alignment expansion in short hypervariable tags (Edgar, 2004). High-quality reads were then clustered based on ⩾95% sequence identity into operational taxonomic units (OTUs) by using the Mothur software (Schloss et al., 2009). Due to the large number of sequences, Mothur was used to create a set of unique sequences, representative of each OTU (at 5% cutoff) via the getotu.rep command. These sequences were searched against public databases with BLAST (Altschul et al., 1997) to verify their affiliation with the respective genes, and detailed sequence analyses were conducted with the ARB software package (Ludwig et al., 2004). Based on amino acids translated from nirS and nosZ genes, these sequences were aligned to our own databases that consist of all publicly available sequences for both the genes as of July 2012 (except for a few environmental Metagenomes). Sequences that were of low quality and could not be aligned properly were omitted. Phylogenetic affiliations of the crust sequences were determined via maximum parsimony with the quick-add search function in ARB against a consensus tree that has been constructed with all sequences in the database based on maximum likelihood, parsimony and distance matrix algorithms. For clarity reasons, only the crust sequences along with their immediate relatives are shown here in this paper.
Results
Denitrification and anammox rates
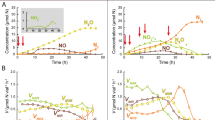
The maximum potential of nitrogenous gas production by denitrification was measured from anoxic slurry incubations with acetylene inhibition technique, while complete denitrification to N2 under more natural conditions was assessed by 15N-tracer experiments with intact crust pieces. The potential rate measurements showed that denitrification started in both the cyanobacteria- and lichen-dominated crusts within the first 2 h of incubation under wet and anaerobic conditions (Figure 1a). The accumulation of N2O in the incubation bottles, indicative of denitrification activity in the crusts, accelerated with time, until nearly linear increases were observed between 4 and 8 h of incubation. Although the temporal dynamics were identical for either crust type, the potential denitrification activity was considerably higher in the cyanobacterial crust than in the lichen crust (Figure 1a, Table 2). The potential rates of total denitrification (that is, including the production of both N2 and N2O) calculated for the 4–8-h time interval were 584±101 and 58±20 μmol N m−2 h−1 for cyanobacterial and lichen crusts, respectively (Table 2). Spontaneous N2O emission from the crusts (that is, N2O emission in the absence of acetylene inhibition) was substantial in either crust type (Figure 1b). N2O emission showed the same temporal dynamics as potential denitrification and reached a rate of 387±143 μmol N m−2 h−1 in the cyanobacterial crust, but was only 31±6 μmol N m−2 h−1 in the lichen crust during the 4–8-h time interval (Table 2). Consequently, the relative share of N2O emission from the total emission of nitrogenous gases was as high as 66% and 53% for the cyanobacterial and lichen crust, respectively. In the killed controls, neither denitrification activity nor N2O emission was observed (Figures 1a and b).
Potential denitrification activity, measured as N2O-N production over time, in slurry incubations of cyanobacterial and lichen crusts amended with 25 μM NO3− and with (a) or without (b) acetylene inhibition of the last step of denitrification. The linear increase in N2O concentration observed between 4 and 8 h of incubation was used to calculate the rates of complete (a) and incomplete (b) denitrification per cm2 of crust. Error bars represent±s.d. (n=6). Additional incubations without nitrate and with autoclaved crust pieces served as negative controls.
The rate measurements with 15NO3− added as a tracer in intact crusts confirmed the occurrence of complete denitrification to N2 (Figure 2). The accumulation of N2 in the incubation bottles (as derived from the accumulation of 29N2, see Materials and methods) followed the same temporal dynamics as observed for the potential rate measurements of denitrification and N2O emission but reached lower concentrations (Figures 1 and 2). Measurement of the background 14NO3− concentrations at the beginning of the incubation (Table 1) unexpectedly revealed that the mole fraction of 15NO3− was as low as 20–26%. Rates of denitrification in intact crusts corrected for these mole fractions were 103±19 and 27±8 μmol N m−2 h−1 for the cyanobacterial and lichen crust, respectively, during the 4–8-h time interval (Table 2). In the killed controls, denitrification activity was not observed (Figure 2).
Denitrification activity, measured as N2-15N production over time, in intact crust pieces amended with 25 μM 15NO3−. The linear increase in N2-15N concentration observed between 4 and 8 h of incubation was used to calculate the rate of denitrification to N2 per cm2 of crust. All incubations were run in triplicates, and autoclaved crust pieces served as negative controls.
The anammox process was not detectable in either crust type using 15NH4+ incubations at mole fractions of 64–65%, as the production of 29N2 was not significantly different from zero for both the living and killed crusts (data not shown).
Ammonia oxidation rates
Measurements of the potential rate of ammonia oxidation showed an immediate recovery in both the crust types after the addition of water and ammonium (Figure 3). The concentration of NO2− increased continuously over time and corresponded to ammonia oxidation rates of 15±2 and 11±5 μmol N m−2 h−1 in the cyanobacterial and lichen crusts, respectively (Table 2). The killed controls did not produce NO2− (Figure 3).
Potential ammonia oxidation activity, measured as NO2− production over time, in slurry incubations of cyanobacterial and lichen crusts amended with 25 μM ammonium sulfate ((NH4)2SO4) and 20 mM sodium chlorate (NaClO3). All incubations were run in triplicates, and autoclaved crust pieces served as negative controls.
Microsensor profiles
Microsensor measurements revealed O2 penetration depths of up to 2 mm in the light in both the crust types, below which the crusts were anoxic (Figure 4a). Oxygen profiles reached steady state after ca. 2 and 3.5 h of water addition and then revealed fluxes of 2.01 and 4.17 mmol O2 m−2 h−1 out of the cyanobacterial and lichen crusts, respectively. In the dark, the crusts turned anoxic within the top 0.5 mm (Figure 4a, only shown for the cyanobacterial crust). The corresponding O2 fluxes into the crusts were 1.20 and 1.25 mmol O2 m−2 h−1 for the cyanobacterial and lichen crusts, respectively.
Depth microprofiles of O2 and N2O measured in intact cyanobacterial crust pieces using microsensors. (a) O2 profiles in the light and in the dark. (b) N2O profiles in a crust piece without the addition of acetylene. The arrow depicts the direction of the increase in N2O concentration with time. (c) N2O profiles in another crust piece without acetylene showing the decrease in N2O concentration after 304 min of measurements. (d) N2O profiles in a crust piece after the addition of acetylene.
N2O profiles from a time-series experiment with cyanobacterial crust pieces revealed a slow increase in N2O concentrations in the first 40 min after addition of water to the crust, followed by a faster progressive increase (Figure 4b). Approximately 3–4 h after wetting, the N2O concentrations reached a maximum, after which it decreased (Figure 4c). At a depth of ca. 3–4 mm, an N2O concentration peak was observed and thus clearly in the anoxic layer of the crust (Figure 4b). The maximum N2O concentration in the crust was as high as 25 μmol l−1. The maximum flux of N2O out of the crust, calculated from the linear concentration gradient in the 1.6–2.4-mm depth interval, was 57.2 μmol N2O-N m−2h−1 (Table 2). The decrease in N2O concentrations was faster than their increase and this transient increase followed by a decrease was observed in several incubations (Figures 4a and b). In acetylene-treated crust pieces, a maximum N2O concentration of 74.3 μmol l−1 was recorded after 68 min (Figure 4d). The shape of the N2O profiles indicates that the layer of N2O production was located at a depth of at least 4–5 mm and thus again clearly in the anoxic layer of the crust (Figure 4d). The maximum flux of N2O, indicating the total flux of gaseous denitrification products, was 153 μmol N2O-N m−2 h−1 in the 3–4 mm depth interval (Table 2). The maximum flux of N2O out of the crust was 74.3 μmol N2O-N m−2 h−1, as calculated from the linear concentration gradient in the 1.6–2.4-mm depth interval (Table 2).
The relative contribution of N2O emission to the total emission of nitrogenous gases was ca. 65% for N2O profiles measured in both the treatments after comparable incubation times (that is, without acetylene: 159 min, with acetylene: 134 min) and calculated for the same depth interval (that is, 1.6–2.4 mm).
Abundance and diversity of functional genes
The functional genes narG, napA and nirS, key to nitrate-reducing and denitrifying bacteria, as well as amoA genes for ammonia-oxidizing bacteria and archaea were detectable in both the cyanobacterial and lichen crusts (Table 3). At the DNA level, the copy numbers of the genes narG and nirS were one order of magnitude higher in the lichen crust (Table 3). The gene napA exhibited comparable estimates in both the crusts. Although the amoA genes of Beta- and Gammaproteobacteria were higher in the lichen crust than in the cyanobacterial crust, the opposite was observed for archaeal amoA (Table 3).
At the mRNA level, narG and nirS were expressed at detectable levels after wetting, as detected by RT-qPCR (Figure 5). The cyanobacterial crust displayed slightly higher transcript number of narG than the lichen crust, whereas the numbers of nirS transcripts were comparable in both the crust types. The narG expression increased with time in cyanobacterial crust, reaching its maximum after 6 h, whereas in lichen crust it increased after 2 h but remained more or less unchanged. The expression of nirS gene showed a general increase with time in both the crust types and reached similar transcript level after 8 h of incubation, although a maximum of 1.49±0.5 × 104 transcripts (g crust)−1 was reached in 2 h in the lichen crust (Figure 5).
A total of 13 645 and 19 361 sequences of nirS and nosZ, respectively, were generated after discarding low-quality reads. The numbers of nirS and nosZ sequences were 1178 and 5717 in the cyanobacterial crust and 12 467 and 13 644 in the lichen crust, respectively (Figure 6). The number of OTUs detected in the cyanobacterial crust was relatively lower than in the lichen crust, which could be due to the variation in sequencing depth. The sequences of nirS genes in the cyanobacterial crusts were assigned to four OTUs based on a 5% nucleic acids sequence identity cutoff, and 99% of these sequences (three OTUs) were phylogenetically affiliated to an uncultured denitrifying bacterium from river sediments (HQ882515), with 61–68% sequence similarity (Figure 6a). The remaining OTU, along with 94% of the nirS sequences of the lichen crust, was most closely affiliated to the known denitrifying bacterium Paracoccus denitrificans, sharing a maximum sequence similarity of 70% (Figure 6a). These sequences represented 59 out of a total of 118 OTUs found in the lichen crust. Other nirS sequences in the lichen crust were affiliated to environmental sequences of uncultured denitrifiers, except for one OTU that represented 1.5% of the total sequences and was related to the nirS gene of Azospirillum (94% similarity). Sequences of the nosZ gene from both the crusts belonged to a total of 574 OTUs and all of them clustered together (Figure 6b). The closest relative to this cluster was a nosZ sequence belonging to Marinobacter hydrocarbonoclasticus, with sequence similarity ⩽66%.
Phylogeny of nirS (a) and nosZ (b) genes in the cyanobacterial and lichen crusts. Phylogenetic affiliations of the crust sequences were determined via maximum parsimony with the quick-add search function in ARB against a consensus tree that has been constructed with all sequences in the database based on maximum likelihood, parsimony and distance matrix algorithms. For clarity, only the crust sequences along with their immediate relatives are shown.
Discussion
We demonstrated that denitrification, and not anammox, is the major nitrogen loss process in crusts from Oman and that N2O gas is emitted during incomplete denitrification. Denitrification rates in crusts from Oman are comparable to those measured in strongly denitrifying freshwater and marine ecosystems (see Table 1 in (Seitzinger, 1988) and references therein), but they were orders of magnitude higher than those reported from other desert crusts (Johnson et al., 2007). Although in our study, the potential denitrification rates of lichen and cyanobacterial crusts, converted to units that match those used in other studies, were equivalent to 805 and 8181 μg N m−2 h−1, respectively, other authors measured <0.3 μg N m−2 h−1 in all the crust types from Colorado Plateau, Sonoran, Chihuahuan and Mojave deserts (Johnson et al., 2007; Strauss and Day, 2012) and 246 μg N m−2 h−1 in crusts from Great Basin Desert (Burns, 1983). The denitrification rates in Omani crusts were still even higher than those measured in non-crusted desert soils from Sonoran Desert (1160 μg N m−2 h−1) and Chihuahuan Desert (315 μg N m−2 h−1; Virginia et al., 1982; Peterjohn and Schlesinger, 1991). When comparing the potential dentrification rates in the Omani crusts to those reported in other studies, it should be noted that there were differences in the incubation conditions. Although our rates were determined in NO3− amended slurries for the time interval 4–8 h, N2O production in Sonoran Desert soils was measured in situ after the addition of distilled water (Virginia et al., 1982), and the measurements in Colorado Plateau, Sonoran, Chihuahuan and Mojave deserts were performed after 4 h of incubation (Johnson et al., 2007; Strauss and Day, 2012). Slurry incubations typically result in higher rates than incubations under diffusive conditions. This explains the higher potential denitrification rates in the studied crusts compared with the rates calculated from microsensor measurments and from 15NO3 incubations. On the other hand, the acetylene inhibition technique used here may underestimate the potential denitrification rates because the acetylene block is alleviated at high sulfide concentrations and acetylene inhibits nitrification (Jones and Knowles, 1992; Sloth et al., 1992). However, in the studied crusts, sulfide could not be measured and NO3 was present in excess and hence denitrification did not depend on NO3 supplied by nitrification.
Incubation experiments, microsensor measurements and gene expression analyses consistently showed that denitrification was activated in both the crust types within 2–4 h after wetting but recovered slightly faster in the cyanobacterial crust. Previous studies, including measurements on the crusts from Oman, showed that other microbial processes also recover rapidly after wetting, with respiration starting immediately within seconds and photosynthesis within minutes to few hours, followed by nitrogen fixation within 2–6 h (Isichei, 1980; Scherer et al., 1984; Issa et al., 2001; Abed et al., 2010). The quick recovery of these processes creates an internal microenvironment suitable for denitrification, including the anoxia generated in deeper crust horizons by intense respiration, the release of photosynthetic organic exudates and the continuous supply of nitrate by nitrifying microorganisms (Garcia-Pichel and Belnap, 1996; Johnson et al., 2007). These are also consistent with the slight initial (∼2 h) time lag in the observed N2 production (Figure 2). The O2 microprofiles in the crusts clearly showed the development of anoxia at depths of 0.5 mm (in the dark) and 2–3 mm (in the light), below which denitrification activity and the concomitant production of N2O could be inferred from N2O microprofiles. The higher denitrification rates measured in slurries and in intact cyanobacterial crusts compared with lichen crusts (Table 2) might be explained by the faster recovery of microbial processes in the cyanobacterial crust. This is reflected in the O2 profiles, where maximum net O2 production was reached after 2 and 3.5 h of wetting in the cyanobacterial and lichen crusts, respectively. Additionally, the somewhat higher NO3− content in the cyanobacterial crust may have enhanced the denitrification activity.
Nitrogen budget: sources and sinks of nitrate
Although the low rates of nitrogen loss processes detected in crusts from Colorado Plateau, Mojave, Sonoran and Chihuahuan deserts (Johnson et al., 2007; Strauss and Day, 2012) seemed to indicate net nitrogen gains in these crust systems, the detected nitrogen losses in the Omani crusts well exceeded nitrogen gains instead. Nitrogen-fixation rates previously measured using acetylene reduction assay in our crusts (that is, 183–258 μg N m−2 h−1, Abed et al., 2010) were comparable with the potential rates of ammonia oxidation (157–208 μg N m−2 h−1) measured in this study. Thus, nitrification obviously does not represent a bottle-neck between N2 fixation and denitrification. However, both the potential dentrification rates and rates measured in intact crusts were at least 2–10 times higher than the N2-fixation and ammonia oxidation rates. This observation implies either that there were external inputs of nitrate fueling denitrification or that there is indeed a net nitrogen loss occurring in the crusts at the instant of sampling, as previously observed in many river, lake and coastal marine ecosystems (Seitzinger, 1988). When the potential rates of denitrification and nitrification in our crusts are compared, it becomes evident that the nitrate supplied by nitrifying bacteria only constitutes a minor fraction of the nitrate reduced by denitrification. Previously, a temporal decoupling of nitrate inputs and losses has been identified, where most of the nitrogen was fixed during precipitation events in winter and fall and is lost through volatilization during summer (Belnap, 2001). However, in hot deserts such as the Omani desert, N inputs and losses are likely to occur simultaneously but with much greater N losses through denitrification and volatilization. As a result, only a very little amount of the newly fixed N will be available for plants and microbial biomass. Besides nitrification, anthropogenic inputs and aeolian deposition combined with limited hydrological export might lead to accumulation of NO3− inside the crusts (Peterjohn and Schlesinger, 1991; Belnap, 2001). In fact, the initial content of readily available NO3− in the crusts used here was substantial (Table 1) and exceeded the experimentally added NO3− by factors of 3.9–4.7. This in-situ, accumulated NO3− alone would sustain denitrification activity at the rates observed here for about 5.3 and 42 h in the cyanobacterial and lichen crusts, respectively. Additionally, the two crust types harbored substantial amounts of intracellularly stored NO3− (Table 1). This intracellular NO3− might sustain denitrification activity for additional 1.5 and 10 h in the cyanobacterial and lichen crust, respectively. It can thus be expected that under in situ conditions, the rates of denitrification will decrease within 1–2 days after wetting to levels sustained by NO3− production through nitrification, unless NO3− is also externally supplied. The validity of these considerations can be tested by studying the seasonal variations of crustal NO3− accumulation and denitrification rates under in situ conditions. Simultaneous rate measurements of all concurrent N-cycling processes by 15N-labeling experiments would also allow a better comparability among N-fluxes and thus more comprehensive nitrogen budgeting (Holtappels et al., 2011).
N2O emission from crusts
Both the incubation experiments and the direct microsensor measurements demonstrated that the wet crusts from Oman are a significant source of N2O. In soils and sediments, N2O can be produced by both nitrification and denitrification (Meyer et al., 2008). However, our experiments suggest that in the two crust types, N2O was entirely produced from incomplete denitrification, as the microsensor profiles clearly showed that N2O was exclusively produced in the anoxic zones of the crusts. N2O concentrations gradually increased but then decreased, as inferred from N2O profiles, which may suggest a delayed induction in the expression of nosZ gene, encoding for the last step of denitrification. Potential rate and microsensor measurements with and without acetylene inhibition showed that N2O gas made up to 54–66% of the total produced gases during denitrification in cyanobacterial and lichen crusts, respectively. Measuring the same N2O yields in both the experimental settings corroborates the validity of our potential rate measurements. The contribution of N2O gas to the total produced nitrogenous gases corresponds to extremely high N2O yields by denitrification, which in other ecosystems are in the order of ⩽1% (Seitzinger, 1988; Bange, 2008). It has previously been suggested that most of the N2O produced in semiarid soils is contributed by fungi (Crenshaw et al., 2008), because they lack the enzyme nitrous oxide reductase (Zhou et al., 2001). However, we measured 13 times higher rates of N2O emission in the cyanobacterial than in the lichen crusts, suggesting a minimal role of the symbiotic fungi in this process. The potential rate of N2O emission in the cyanobacterial crust was as high as 387 μmol m−2 h−1, whereas the N2O flux derived from the microsensor profiles was only 52–57 μmol m−2 h−1. This difference in rates can be attributed to the generally higher reaction rates in slurry incubations compared with incubations in which the vertical stratification remains intact and solute transport is diffusion-limited. Regardless of the technique used, the measured N2O emission rates were still higher than those of other desert crusts and soils. N2O was emitted from soils of the Sonoran Desert at fluxes ranging from 0.4 to 1.88 μmol N2O-N m−2 h−1 (Guilbault and Mathias, 1998) and in Great Basin soils at a rate of 0.005 μmol N2O-N m−2 h−1(Mummey et al., 1994); however, it was unclear if these soils contained crusts. Field measurements of gaseous losses in wetted crusts from Utah revealed N2O emission at a rate of 1.82–3.57 μmol N2O-Nm−2 h−1 (Belnap, 2001).
Denitrifying bacteria
The detection of functional genes by qPCR, RT-qPCR and pyrosequencing indicates that denitrifying bacteria are present in both the crusts, thus supporting the observed denitrification activity. Despite the seemingly higher gene abundance of narG, napA and nirS in the lichen crust, the expression of narG was considerably higher in the cyanobacterial crust, which also showed a more steady increase in nirS expression during the 8-h incubation. These might be the result of a somewhat higher NO3− content in the cyanobacterial crust that likely has enhanced denitrification activity (by >10-fold) relative to the lichen crust. These gene expression patterns are consistent with the greater denitrification rates measured in cyanobacterial crust compared with the lichen crust. Although the possibility of residual mRNA in dry crusts cannot be fully eliminated especially in view of the detection of narG and nirS transcription after merely 5 min of wetting, the fact that narG and nirS transcripts increased substantially in both the crusts (except for nirS mRNA in lichen crust) with time indicates active gene expression during the incubation periods. The strong and rapid expression of narG and nirS genes corroborates the activities of denitrification at least in the first two steps: nitrate reduction to nitrite and then to nitric oxide. Nitrite may also be reduced by other microorganisms using the copper-containing nitrite reductase (nirK), as nirS and nirK have never been found in the same cell (Zumft, 1997). Despite the attempts made to amplify and sequence nirK via TEFAP, the obtained nirK sequences unfortunately had to be excluded from further analysis because of their low quality. Nevertheless, it should also be noted that nirK has also been found in archaeal and bacterial ammonia oxidizers, as well as an anammox bacterium, as such it is not an ideal biomarker gene for denitrifiers (Cantera and Stein, 2007; Bartossek et al., 2010; Hira et al., 2012).
Complete denitrification through the conversion of N2O to N2 in the crusts from Oman was confirmed by the detection of nosZ genes. All these nosZ sequences did not branch with known denitrifying bacteria and only had <65% similarity to the closest relatives, indicating their novelty. Similarly, most of the nirS sequences (99%) retrieved from the cyanobacterial crust and to a lesser extent (4.5%) from the lichen crust were related to uncultured denitrifying bacteria, thus indicating unique, yet to be cultivated, nitrite reducing/denitrifying communities within these crusts. On the other hand, the majority of nirS sequences in the lichen crust were most closely affiliated to the well-known denitrifiers P. denitrificans and Azospirillum, of the Alphaproteobacteria group. The latter, in particular, have previously been detected in the same crusts via pyrosequencing analysis of the 16S rRNA gene (Abed et al., 2010). P. denitrificans is mainly found in soils and sewage treatment plants and is nutritionally versatile with a heterotrophic growth mode under aerobic conditions with oxygen and under anaerobic conditions with nitrate as an electron acceptor (Baker et al., 1998). An interesting feature of this organism is its ability to perform denitrification, even in the presence of oxygen (Davies et al., 1989). This makes this organism suitable to survive in compact crusts in which it has to cope with fluctuating conditions of high oxygen concentration due to cyanobacterial photosynthesis during the day and anoxia in the dark.
Environmental implications
Due to the large global expanse of arid deserts (30–40% of the Earth’s surface), and the Arabian Desert in particular, our findings may have significant environmental implications. Our rate measurements indicate that desert crusts produce and emit N2O gas immediately after wetting and thereby might contribute to the greenhouse effect, the destruction of the protecting ozone layer in the stratosphere and rainfall acidity (Dickinson and Cicerone, 1986; Cicerone, 1987). However, the magnitude of these effects can only be judged after obtaining information on the coverage of crusts, the frequency of precipitation events and how long the crusts retain their moisture and the rates of denitrification and nitrous oxide emission under field conditions. It can further be speculated that the high loss of fixed nitrogen through denitrification relative to nitrogen inputs in the Arabian Desert may significantly exacerbate the fertility of the desert soil. This nitrogen cycling pattern in the Arabian Desert poses a serious threat to desert life, and nitrogen losses may even increase with the observed changes in temperature and precipitation patterns due to global climate changes (McCalley and Sparks, 2009).
References
Abed RMM, Kharusi SA, Schramm A, Robinson MD . (2010). Bacterial diversity, pigments and nitrogen fixation of biological desert crusts from the Sultanate of Oman. FEMS Microbiol Ecol 72: 418–428.
Allan JA, Warren A . (1993) Deserts: The Encroaching Wilderness: A World Conservation Atlas. Oxford University Press: New York, NY, USA.
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W et al (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402.
Andersen K, Kjaer T, Revsbech NP . (2001). An oxygen insensitive microsensor for nitrous oxide. Sens Actuators B Chem 81: 42–48.
Angel R, Matthies D, Conrad R . (2011). Activation of methanogenesis in arid biological soil crusts despite the presence of oxygen. PLoS One 6: 1–7.
Austin AT, Yahdjian L, Stark JM, Belnap J, Porporato A, Norton U et al (2004). Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 141: 221–235.
Baker SC, Ferguson SJ, Ludwig B, Page MD, Richter OM, Spanning RJ . (1998). Molecular genetics of the genus Paracoccus: metabolically versatile bacteria with bioenergetic flexibility. Microbiol Mol Biol Rev 62: 1046–1078.
Bange HW . (2008). Gaseous nitrogen compounds (NO, N2, NH3) in the ocean. In: Capone DG, Bronk DA, Mulholland MR, Carpenter EJ, (eds). Nitrogen in the Marine Environment. Elsevier Press: Amsterdam, The Netherlands, pp 51–94.
Barger NN, Belnap J, Ojima D, Mosier A . (2005). NO gas loss from N-fixing biologically crusted soils in Canyonlands National Park, Utah. Biogeochemistry 75: 373–391.
Bartossek R, Nicol GW, Lanzen A, Klenk H-P, Schleper C . (2010). Homologues of nitrite reductases in ammonia-oxidizing archaea: diversity and genomic context. Environ Microbiol 12: 1075–1088.
Belnap J . (2001). Factors influencing nitrogen fixation and nitrogen release in biological soil crusts. In: Belnap J, Lange OL, (eds). Biological Soil Crusts: Structure, Function, and Management. Springer-Verlag: Berlin, Germany, pp 241–261.
Belnap J . (2002). Nitrogen fixation in biological soil crusts from southeast Utah, USA. Biol Fertil Soils 35: 128–135.
Belnap J . (1995). Recovery of nitrogenase activity in cyanobacterial-lichen soils crusts of the Great Basin and the Colorado Plateau. In: The Transdisciplinary Nature of Ecology 1995; Snowbird, Utah, USA.
Belser LW, Mays EL . (1980). Specific inhibition of nitrite oxidation by chlorate and its use in assessing nitrification in soils and sediments. Appl Environ Microbiol 39: 505–510.
Billings SA, Schaeffer SM, Evans RD . (2003). Nitrogen fixation by biological soil crusts and heterotrophic bacteria in an intact Mojave Desert ecosystem with elevated CO2 and added soil carbon. Soil Biol Biochem 35: 643–649.
Braman RS, Hendrix SA . (1989). Nanogram nitrite and nitrate determination in environmental and biological materials by vanadium(III) reduction with chemiluminescence detection. Anal Chem 61: 2715–2718.
Burns SJ . (1983). Nitrogen fixation and transformations in cryptogamic soil crusts as affected by disturbance. MSc thesis Brigham Young University: Provo, UT, USA.
Cantera JJL, Stein LY . (2007). Molecular diversity of nitrite reductase genes (nirK) in nitrifying bacteria. Environ Microbiol 9: 765–776.
Cicerone RJ . (1987). Changes in stratospheric zone. Science 237: 35–42.
Crenshaw CL, Lauber C, Sinsabaugh RL, Stavely LK . (2008). Fungal control of nitrous oxide production in semiarid grassland. Biogeochemistry 87: 17–27.
Davies KJP, LLoyd D, Boddy L . (1989). The effect of oxygen on denitrification in Paracoccus denitrificans and Pseudomonas aeruginosa. J Gen Microbiol 135: 2445–2451.
Dickinson RE, Cicerone RJ . (1986). Future global warming from atmospheric trace gases. Nature 319: 109–115.
Dowd SE, Callaway TR, Wolcott RD, Sun Y, McKeehan T, Hagevoort RG et al (2008b). Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP). BMC Microbiol 8: 125.
Dowd SE, Sun Y, Wolcott RD, Domingo A, Carroll JA . (2008a). Bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP) for microbiome studies: bacterial diversity in the ileum of newly weaned Salmonella-infected pigs. Foodborne Pathog Dis 5: 459–472.
Edgar RC . (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797.
Flanagan DA, Gregory LG, Carter JP, Karakas-Sen A, Richardson DJ, Spiro S . (1999). Detection of genes for periplasmic nitrate reductase in nitrate respiring bacteria and in community DNA. FEMS Microbiol Lett 177: 263–270.
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB . (2005). Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci USA 102: 14683–14688.
Gao K, Qui B, Xia J, Yu A . (1998). Light dependency of the photosynthetic recovery of Nostoc flagelliforme. J Appl Phycol 10: 51–53.
Garbauer RL, Ehleringer JR . (2000). Water and nitrogen uptake patterns following moisture pulses in a cold desert community. Ecology 81: 1415–1424.
Garcia-Pichel F, Belnap J . (1996). Microenvironments and microscale productivity of cyanobacterial desert crusts. J Phycol 32: 774–782.
Guilbault MR, Mathias AD . (1998). Emissions of N2O from Sonoran desert and effluent-irrigated grass ecosystems. J Arid Environ 38: 87–98.
Hira D, Toh H, Migita CT, Okubo H, Nishyama T, Hattori M et al (2012). Anammox organism KSU-1 expresses a nirK-type copper-containing nitrite reductase instead of a nirS-type with cytochrome cd. FEBS Lett 586: 1658–1663.
Holtappels M, Lavik G, Jensen MM, Kuypers MMM . (2011). 15N-labeling experiments to dissect the contributions of heterotrophic denitrification and anammox to nitrogen removal in the OMZ waters of the ocean. Methods Enzymol 486: 223–251.
Humbert S, Tarnawski S, Fromin N, Mallet M-P, Aragno M, Zopfi J . (2010). Molecular detection of anammox in bacteria in terrestrial ecosystems: distribution and diversity. ISME J 4: 450–454.
Isichei AO . (1980). Nitrogen fixation by blue-green algal soil crusts in Nigerian savanna. In: Rosswall T, (ed). Nitrogen Cycling in West African Ecosystems. Reklam and Katalogtryck: Uppsala, Sweden, pp 191–198.
Issa OM, Stal LJ, Défarge C, Couté A, Trichet J . (2001). Nitrogen fixation by microbial crusts from desiccated Sahelian soils (Niger). Soil Biol Biochem 33: 1425–1428.
Johnson SL, Budinoff CR, Belnap J, Garcia-Pichel F . (2005). Relevance of ammonium oxidation within biological soil crust communities. Environ Microbiol 7: 1–12.
Johnson SL, Neuer S, Garcia-Pichel F . (2007). Export of nitrogenous compounds due to incomplete cycling within biological soil crusts of arid lands. Environ Microbiol 9: 680–689.
Jones AM, Knowles R . (1992). Sulfide alleviation of acetylene inhibition of nitrous oxide reduction by Flexibacter canadensis. Can J Microbiol 38: 143–148.
Kamp A, de Beer D, Nitsch JL, Lavik G, Stief P . (2011). Diatoms respire nitrate to survive dark and anoxic conditions. Proc Natl Acad Sci USA 108: 5649–5654.
Kempers AJ, Kok CJ . (1989). Re-examination of the determination of ammonium as the indophenol blue complex using salicylate. Anal Chim Acta 221: 147–155.
Kuypers MMM, Lavik G, Woebken D, Schmid M, Fuchs BM, Amann R et al (2005). Massive nitrogen loss from the Benguela upwelling system through anaerobic ammonium oxidation. Proc Natl Acad Sci USA 102: 6478–6483.
Kuypers MMM, Olav Sliekers A, Lavik G, Schmid M, Jørgensen BB, Gijs Kuenen J et al (2003). Anaerobic ammonium oxidation by anammox bacteria in the Black Sea. Nature 422: 608–611.
Lam P, Kuypers MMM . (2011). Microbial nitrogen cycling processes in oxygen minimum zones. Ann Rev Mar Sci 3: 317–345.
Lam P, Lavik G, Jensen MM, Vossenberg J, Schmid M, Woebken D et al (2009). Revising the nitrogen cycle in the peruvian oxygen minimum zone. Proc Natl Acad Sci USA 106: 4752–4757.
Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar et al (2004). ARB: a software environment for sequence data. Nucleic Acids Res 32: 1363–1371.
McCalley CK, Sparks JP . (2009). Abiotic gas formation drives nitrogen loss from a desert ecosystem. Science 326: 837–840.
McNeill A, Unkovich M . (2007). The Nitrogen cycle in terrestrial ecosystems. In: Marschner P, Rengel Z, (eds). Nutrient Cycling in Terrestrial Ecosystems. Springer Verlag: Berlin, Heidelberg, Germany, pp 37–64.
Meyer RL, Allen DE, Schmidt S . (2008). Nitrification and denitrification as sources of sediment nitrous oxide production: a microsensor approach. Mar Chem 110: 68–76.
Michotey V, Meijean V, Bonin P . (2000). Comparison of methods for quantification of cytochrome cd1-denitrifying bacteria in environmental marine samples. Appl Environ Microbiol 66: 1564–1571.
Mummey DL, Smith JL, Bolton H . (1994). Nitrous oxide flux from a shrub-steppe ecosystem: sources and regulation. Soil Biol Biochem 26: 279–286.
Muyi C, Caldwell MM . (1997). A large ephemeral release of nitrogen upon wetting of dry soil and corresponding root responses in the field. Plant Soil 191: 299.
Nielsen LP . (1992). Denitrification in sediment determined from nitrogen isotope pairing. FEMS Microbiol Lett 86: 357–362.
Peterjohn WT, Schlesinger WH . (1991). Factors controlling denitrification in a Chihuahuan Desert ecosystem. Soil Sci Soc Am J 55: 1694–1701.
Philippot L, Piutti S, Martin-Laurent F, Hallet S, Germon JC . (2002). Molecular analysis of the nitrate-reducing community from unplanted and maize-planted soils. Appl Environ Microbiol 68: 6121–6128.
Polerecky L, Bachar A, Schoon R, Grinstein M, Jørgensen BB, de Beer D et al (2007). Contribution of Chloroflexus respiration to oxygen cycling in a hypersaline microbial mat from Lake Chiprana, Spain. Environ Microbiol 9: 2007–2024.
Poth M . (1986). Dinitrogen production from nitrite by a Nitrosomonas isolate. Appl Environ Microbiol 52: 957–959.
Purkhold U, Wagner W, Timmermann G, Pommerening-Röser A, Koops H-P . (2000). Phylogeny of all recognized species if ammonia oxidizers based on comparative 16S rRNA and amoA sequence analysis: implications for molecular diversity surveys. Appl Environ Microbiol 66: 5368–5382.
Qiu B, Gao K . (1999). Dried field populations of Nostoc flagelliforme (Cyanophyceae) require exogenous nutrients for their photosynthetic recovery. J Appl Phycol 11: 535–541.
Qiu BS, Zhang AH, Liu ZL, Gao KS . (2004). Studies on the photosynthesis of the terrestrial cyanobacterium Nostoc flagelliforme subjected to desiccation and subsequent rehydration. Phycologia 43: 521–528.
Revsbech NP . (1989). An oxygen microsensor with a guard cathode. Limnol Oceanogr 34: 474–478.
Ritchie GAF, Nicholas DJD . (1972). Identification of nitrous oxide produced by oxidative and reductive processes in Nitrosomonas europaea. Biochem J 126: 1181–1191.
Rotthauwe J-H, Witzel k-P, Liesack W . (1997). The ammonia monooxygenase strcutural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia oxidizing populations. Appl Environ Microbiol 63: 4704–4712.
Santoro AE, Buchwald C, McIlvin MR, Casciotti KL . (2011). Isotopic signature of N2O produced by marine ammonia-oxidizing archaea. Science 333: 1282–1285.
Scherer S, Ernst A, Chen T-W, Böger P . (1984). Rewetting of drought-resistant blue green algae: time course of water uptake and reappearance of respiration, photosynthesis, and nitrogen fixation. Oecologia 62: 418–423.
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB et al (2009). Introducing mothur: open source, platform-independent, community supported software for describing and comparing microbial communities. Appl Environ Microbiol 75: 7537–7541.
Schmidt I, van Spanning RJM, Jetten MSM . (2004). Denitrification and ammonia oxidation by Nitrosomonas europaea wild-type, and NirK- and NorB-deficient mutants. Microbiol 150: 4107–4114.
Seitzinger SP . (1988). Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance. Limnol Oceanogr 33: 702–724.
Sloth NP, Nielsen LP, Blackburn TH . (1992). Nitrification in sediment cores measured with acetylene inhibition. Limnol Oceanogr 37: 1108–1112.
Stief P, de Beer D . (2006). Probing the microenvironment of freshwater sediment macrofauna: implications of deposit-feeding and bioirrigation for nitrogen cycling. Limnol Oceanogr 51: 2538–2548.
Strauss SL, Day TA . (2012). Nitrogen cycling in desert biological soil crusts across biogeographic regions in the Southwestern United States. Biogeochemistry 108: 171–182.
Sørensen J . (1978). Occurrence of nitric and nitrous oxides in a coastal marine sediment. Appl Environ Microbiol 36: 809–813.
Ullman WJ, Aller RC . (1982). Diffusion coefficients in nearshore marine sediments. Limnol Oceanogr 27: 552–556.
Veluci RM, Neher DA, Weicht TR . (2006). Nitrogen fixation and leaching of biological soil crust communities in mesic temperate soils. Microb Ecol 51: 189–196.
Virginia RA, Jarrell WM, Franco-Vizcaino E . (1982). Direct measurement of denitrification in a Priosopis (Mesquite) dominated Sonoran Desert ecosystem. Oecologia 53: 120–122.
Ward BB, Devol AH, Rich JJ, Chang BX, Bulow SE, Naik H et al (2009). Denitrification as the dominant nitrogen loss process in the Arabian Sea. Nature 461: 78–82.
Wrage N, Velthof GL, Beusichem MLV, Oenema O . (2001). Role of nitrifier denitrificaiton in the production of nitrous oxide. Soil Biol Biochem 33: 1723–1732.
Yang F, Troncy E, Francoeur M, Vinet B, Vinay P, Czaika G et al (1997). Effects of reducing reagents and temperature on conversion of nitrite and nitrate to nitric oxide and detection of NO by chemiluminescence. Clin Chem 43: 657–662.
Yeager CM, Kornosky JL, Housman DC, Grote EE, Belnap J, Kuske C . (2004). Diazotrophic community structure and function in two successional stages of biological soil crusts from the Colorado Plateau and Chihuahuan desert. Appl Environ Microbiol 70: 973–983.
Zhou A, Takaya N, Sakairi MAC, Shoun H . (2001). Oxygen requirement for denitrification by the fungus Fusarium oxysporum. Arch Microbiol 175: 25.
Zumft WG . (1997). Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61: 533–616.
Acknowledgements
We thank the Hansewissenschaftskolleg, Institute for Advanced Studied in Delmenhorst, Germany for supporting RA. We also thank the technicians of the Microsensor Group of the Max Planck Institute for Marine Microbiology in Bremen for microsensor construction. This research was funded by The Research Council (TRC) of Oman (Grant No. RC/SCI/BIOL/11/01) and the Max-Planck Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Abed, R., Lam, P., de Beer, D. et al. High rates of denitrification and nitrous oxide emission in arid biological soil crusts from the Sultanate of Oman. ISME J 7, 1862–1875 (2013). https://doi.org/10.1038/ismej.2013.55
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2013.55
Keywords
This article is cited by
-
Water-driven microbial nitrogen transformations in biological soil crusts causing atmospheric nitrous acid and nitric oxide emissions
The ISME Journal (2022)
-
Nitrous oxide emissions and maize yield as influenced by nitrogen fertilization and tillage operations in upland soil
Applied Biological Chemistry (2021)
-
Origin of Cr in Alluvial and Aeolian Sediments and Ultramafic Rocks in Sultanate of Oman: Magnetic Fractionation and Sunlight Effect
Bulletin of Environmental Contamination and Toxicology (2021)
-
Soil total organic carbon/total nitrogen ratio as a key driver deterministically shapes diazotrophic community assemblages during the succession of biological soil crusts
Soil Ecology Letters (2021)
-
Bacteria and fungi differentially contribute to carbon and nitrogen cycles during biological soil crust succession in arid ecosystems
Plant and Soil (2020)