Abstract
Phytoplankton growth can be limited by numerous inorganic nutrients and organic growth factors. Using the subarctic diatom Attheya sp. in culture studies, we examined how the availability of vitamin B12 and carbon dioxide partial pressure (pCO2) influences growth rate, primary productivity, cellular iron (Fe), cobalt (Co), zinc (Zn) and cadmium (Cd) quotas, and the net use efficiencies (NUEs) of these bioactive trace metals (mol C fixed per mol cellular trace metal per day). Under B12-replete conditions, cells grown at high pCO2 had lower Fe, Zn and Cd quotas, and used those trace metals more efficiently in comparison with cells grown at low pCO2. At high pCO2, B12-limited cells had ∼50% lower specific growth and carbon fixation rates, and used Fe ∼15-fold less efficiently, and Zn and Cd ∼3-fold less efficiently, in comparison with B12-replete cells. The observed higher Fe, Zn and Cd NUE under high pCO2/B12-replete conditions are consistent with predicted downregulation of carbon-concentrating mechanisms. Co quotas of B12-replete cells were ∼5- to 14-fold higher in comparison with B12-limited cells, suggesting that >80% of cellular Co of B12-limited cells was likely from B12. Our results demonstrate that CO2 and vitamin B12 interactively influence growth, carbon fixation, trace metal requirements and trace metal NUE of this diatom. This suggests the need to consider complex feedback interactions between multiple environmental factors for this biogeochemically critical group of phytoplankton in the last glacial maximum as well as the current and future changing ocean.
Similar content being viewed by others
Introduction
Marine phytoplankton account for about half of global primary production (Behrenfeld and Falkowski, 1997), and depend on the availability of dissolved inorganic carbon, major nutrients and trace elements. Many eukaryotic phytoplankton also require a number of organic growth cofactors including cobalamin—vitamin B12 (Provasoli and Carlucci, 1974)—a cobalt (Co)-containing molecule synthesized by many, but not all, bacteria and archaea (Rodionov et al., 2003). About half of cultured eukaryotic phytoplankton species, including many harmful algal bloom dinoflagellates, are unable to synthesize B12 and thus require an exogenous supply (Provasoli and Carlucci, 1974; Croft et al., 2005; Tang et al., 2010). The renewed attention to B12 over the past decade has led to numerous field studies that have demonstrated that vitamin B12 is a limiting factor in a variety of marine ecosystems, including temperate and polar coastal waters (Sañudo-Wilhelmy et al., 2006; Gobler et al., 2007), iron (Fe)-limited/high-nutrient regimes of the Southern Ocean (Bertrand et al., 2007) and the high-nitrate, low-chlorophyll (HNLC) Gulf of Alaska (Koch et al., submitted).
Another factor potentially affecting phytoplankton growth that has recently received attention is the availability of dissolved inorganic carbon in the context of ocean acidification (reviewed by Hutchins et al., 2009). Past glacial–interglacial variability in carbon dioxide partial pressure (pCO2) based on paleoclimate proxies (Petit et al., 1999) and the future predicted anthropogenic rise in pCO2 (Solomon et al., 2007) could influence phytoplankton physiology and elemental requirements. For instance, a higher CO2 availability could alleviate high metabolic energy and trace metal requirements of the carbon-concentrating mechanisms that supply CO2 for photosynthesis (Raven, 1991; Morel et al., 1994).
Despite the global importance of both vitamin B12 and pCO2 to phytoplankton metabolic pathways, virtually nothing is known about the possible interactions between these two critical factors. We tested the effects of B12 and CO2 availability on specific growth rates, primary productivity and the requirements of major nutrients (C, N and P) and Fe, as well as other trace metals (zinc (Zn), Co and cadmium (Cd)) in steady-state semi-continuous cultures of Attheya sp., a centric diatom isolated from the Bering Sea (an Fe-limited HNLC region; Leblanc et al., 2005). Attheya sp. belongs to the family Chaetocerotaceae, and was previously classified in the genus Chaetoceros (Crawford et al., 2000), a biogeochemically important algal group that dominates blooms in coastal upwelling regions and in mesoscale and bottle Fe addition experiments (Hutchins and Bruland, 1998; de Baar et al., 2005).
Materials and methods
Unialgal axenic Attheya sp. (CCMP207) stock cultures, isolated from the Bering Sea, were obtained from the Provasoli-Guillard Culture Collection of Marine Phytoplankton (West Boothbay Harbor, Maine, USA) and grown in microwave-sterilized media prepared from 0.2 μm-filtered natural seawater (collected using trace metal clean techniques). Media had Aquil concentrations of nitrate, phosphate and silicic acid with additions of 5 μM EDTA, 451 nM Fe, 80 nM Zn, 50 nM Co and no added Cd (Price et al., 1988/89). Stock cultures were incubated at 3 °C with a 12 h:12 h light–dark cycle and an incident photon flux density of 80 μmol photons m−2 s−1. Although B12 auxotrophy has not been evaluated for Attheya sp., various centric diatoms including species from the same family, Chaetocerotaceae, have been found to require vitamin B12 (Provasoli and Carlucci, 1974). Cultures were verified to be axenic by staining subsamples with 4′,6-diamidino-2-phenolindole, followed by bacterial counts using epifluorescence microscopy directly before the final sampling.
Experimental treatments included trace metal clean vitamin B12 additions of 500 and 10 ng l−1 (370 and 7 pM) for the B12-replete and B12-limited cultures, respectively. The 7 pM vitamin B12 was used for B12-limited cultures because steady-state growth rates at that concentration were about half of maximum growth rate. Vitamin and nutrient stocks were cleaned of trace metals before use by passing them through a column packed with Chelex-100 (Bio-Rad, Hercules, CA, USA; Price et al., 1988/89). For both B12 conditions, triplicate acid-washed polycarbonate bottles were equilibrated with commercially prepared air:CO2 mixtures at three different CO2 concentrations: 200, 370 and 670 p.p.m. pCO2 (see below). In-line HEPA filters were used to avoid trace metal and bacterial contamination from the gas tanks or lines.
Trace metal clean semi-continuous culturing methods were used during acclimation periods and steady-state growth. Final sampling was carried out following 4–8 weeks of semi-continuous incubation after statistically invariant growth rates were recorded for at least three consecutive transfers. Cultures were grown semi-continuously to maintain cells in the exponential growth phase to avoid bias resulting from sampling during different growth phases in different experimental treatments. All medium handling, culturing and manipulation used careful trace metal clean methods under class 100 conditions.
Seawater medium pCO2 in the experimental bottles was calculated throughout the experiment using measurements of pH and total dissolved inorganic carbon according to Dickson and Goyet (1994). To ensure that CO2 levels remained constant during growth, the pH in each bottle was monitored daily using a microprocessor pH meter, calibrated with pH 4, 7 and 10 buffer solutions. Total dissolved inorganic carbon was measured via coulometry (model CM 140, UIC, Joliet, IL, USA). The calculated pCO2 values (using CO2SYS; http://www.cdiac.ornl.gov/ftp/co2sys/CO2SYS_calc_XLS) from the final day of the experiment were 201±13, 367±21 and 671±31 p.p.m. (Table 1); these treatments are referred to using rounded-off values of 200, 370 and 670 p.p.m. pCO2.
Primary production was measured in triplicate using 24 h incubations with 3.7 kBq ml−1 H14CO3 under the appropriate experimental growth conditions of light and temperature for each treatment, followed by filtration and scintillation counting. Aliquots for analysis of particulate organic C and N were filtered onto pre-combusted 25 mm GF/F filters (Whatman, Maidstone, UK) and analyzed using a 4010 CHNS Elemental Combustion System (Costech, Valencia, CA, USA). Samples for particulate organic phosphorous were processed and analyzed spectrophotometrically. Details for these methods can be found in Fu et al. (2005, 2007; and references therein).
Particulate samples for trace metal analysis were filtered onto acid-washed 3-μm-pore-size polycarbonate filters (Millipore, Billerica, MA, USA), rinsed with oxalate reagent to remove extracellular trace metals (Tovar-Sanchez et al., 2003), and Fe, Zn, Co and Cd were determined with a magnetic sector-field high-resolution inductively coupled plasma mass spectrometer (ICPMS) (Element 2, Thermo, Waltham, MA, USA). Procedural filter blanks were also subjected to the same storage, digestion, dilution and analysis processes, and these blank values were subtracted from sample measurements (Sañudo-Wilhelmy et al., 2001). The digestion protocol consisted of sequential additions of ultrapure HCl, HNO3 and HF (Omnitrace Ultra; VWR, Westchester, PA, USA) and heating to 100 °C (Eggimann and Betzer, 1976).
The measured concentrations of particulate elements (C, N, Fe, Co, Zn and Cd) were normalized to P. Previous studies have demonstrated that phytoplankton cellular P quotas are usually not affected by changing pCO2 (see review by Hutchins et al., 2009). Cellular elemental ‘quota’ or ‘content’ are reported in this article interchangeably with ‘requirement’, although we acknowledge that cellular quotas could possibly be elevated by luxury uptake and/or storage of trace metals (for instance, Fe luxury uptake/storage; Sunda and Huntsman, 1995a; Marchetti et al., 2009). Trace metal:C ratios were used in conjunction with specific growth rates to calculate net use efficiencies (NUEs; specific growth rate divided by trace metal:C ratio), representing how efficiently trace metals are used by cells for growth and C fixation (Raven, 1991). This treatment implicitly circumvents concerns about distinguishing trace metal ‘content’ from ‘requirements’, as it quantitatively links the trace metal quota of the cells to growth and photosynthetic rates. Significant differences in growth parameters, nutrient/trace metal quotas and trace metal NUE were tested using two-way analysis of variance and Student's t-test (SigmaStat, Ashburn, VA, USA).
Results
Effect of B12 and pCO2 variability on specific growth rates and primary production
Specific growth rates (Figure 1a) and 14C-based primary productivity (Figure 1b) of B12-limited cells (grown at 7 pM B12) were significantly lower (∼40–60% lower) than B12-replete cells (grown at 370 pM B12) at all three pCO2 concentrations (200, 370 and 670 p.p.m. pCO2; P<0.05). Specific growth rates and primary productivity of B12-replete cells were positively correlated with pCO2 (r2=0.99 and 0.97, respectively), and were ∼30–40% higher at 670 p.p.m. compared with 200 p.p.m. pCO2 (P>0.05) (Figure 1). In B12-limited treatments, specific growth rates and primary productivity were not significantly different among CO2 treatments (P>0.05) (Figure 1).
Effect of B12 and pCO2 variability on elemental ratios
Cellular molar C:P (Figure 2a) and N:P (Figure 2b) ratios of B12-replete cells were significantly higher at 670 p.p.m. in comparison with the other two lower pCO2 treatments (P<0.05). Both C:P and N:P of cells from 670 p.p.m. pCO2 treatments were also significantly lower in B12-limited cells in comparison with B12-replete cells (P<0.05). There were no significant differences in C:P or N:P ratios between the three pCO2 treatments in B12-limited cultures (Figure 2; P>0.05).
Fe quotas (mmol Fe:mol P) of B12-replete cells were negatively correlated with pCO2 (r2=0.92), and Fe:P ratios at 370 p.p.m. (24±11) and 670 p.p.m. (14±1) were ∼40% and ∼70% lower, respectively, in comparison with Fe:P at 200 p.p.m. pCO2 (41±13; P<0.05; Figure 3a). For B12-limited cells, Fe:P was positively correlated with pCO2 (r2=0.93), and Fe:P of cells grown at 200 p.p.m. was ∼50% and ∼60% lower than that of cells at 370 and 670 p.p.m. pCO2, respectively (P<0.05). FeNUE (mol C fixed per mol Fe per day) of B12-replete cells were comparable with FeNUE of B12-limited cells at 200 p.p.m. pCO2, but FeNUE of B12-replete cells were ∼4-fold and ∼15-fold greater than B12-limited cells at 370 and 670 p.p.m. pCO2, respectively (P<0.05; Figure 4a).
Co:P ratios (mmol:mol) in B12-replete treatments were negatively correlated with pCO2 (r2=0.99) (Figure 3b). In B12-limited treatments, Co:P ratios were an order of magnitude lower ranging from 0.001 to 0.005 mmol:mol and were significantly lower in comparison with B12-replete treatments at all CO2 treatments (P<0.05) (Figure 3b). Co:P in B12-limited treatments were positively correlated with pCO2 (r2=0.73), and Co:P of cells grown at 670 p.p.m. pCO2 were significantly higher than cells grown at 200 p.p.m. pCO2 (P<0.05; Figure 3b). CoNUE in both B12-replete and B12-limited treatments were comparable at 670 p.p.m. pCO2, but CoNUE of B12-limited treatments were ∼5-fold and ∼15-fold greater in comparison with B12-replete treatments at 370 and 200 p.p.m. pCO2 (P<0.05), respectively (Figure 4b).
Zn:P and Cd:P ratios (mmol:mol) of B12-replete and B12-limited treatments displayed a similar negative and positive correlation with pCO2, respectively, as observed for Fe:P (Figures 3c and d). ZnNUE and CdNUE were approximately threefold higher in 670 p.p.m. pCO2/B12-replete cells when compared with 670 p.p.m. pCO2/B12-limited cells (P<0.05), but were not significantly different between B12 treatments at 200 and 370 p.p.m. pCO2 (P>0.05) (Figures 4c and d).
Discussion
Growth rates, primary productivity and major elemental ratios
Phytoplankton B12 limitation has been previously observed in natural phytoplankton assemblages in the US East Coast waters for the >5 μm size class as well as for specific taxa, such as diatoms and dinoflagellates (Sañudo-Wilhelmy et al., 2006; Gobler et al., 2007). B12 limitation or B12/Fe colimitation has also been documented in phytoplankton communities in HNLC areas of the Southern Ocean and the Gulf of Alaska (Panzeca et al., 2006; Bertrand et al., 2007; Koch et al., submitted). Although Attheya sp. (CCMP207) has not been previously tested for dependence on vitamin B12, our results (Figure 1a) clearly demonstrate growth rate limitation at B12 concentrations similar to those measured in the coastal and open ocean (0.2–7 pM; Panzeca et al., 2009) and in the range of previously reported B12 half-saturation constants reported for other diatoms (∼0.1–0.8 pM; reviewed by Droop, 2007). Specific growth rates and primary productivity were significantly lower under B12 limitation at all pCO2 concentrations (Figure 1). Similar reductions in growth rate and primary production by B12 limitation have previously been reported for other diatoms in culture (Carlucci and Silbernagel, 1969; Swift and Taylor, 1974). In our semi-continuous culture design, regular dilutions were used to prevent the cultures from entering stationary phase, thus prohibiting any evaluation of biomass yield limitation. Our experiments therefore examined rate limitation only, and future work using batch culture experiments is needed for direct comparisons with B12 limitation of biomass yield (for example, Droop, 1957).
Furthermore, consistent with the influence of B12 concentrations on growth and carbon assimilation rates, C:P and N:P ratios of B12-limited cells were significantly lower in comparison with B12-replete replicates at 670 p.p.m. pCO2 (Figure 2). Potential changes in phytoplankton productivity, C:P and N:P at elevated pCO2 could have large implications for global nutrient cycles and the ocean's biological pump (Riebesell et al., 2007; Hutchins et al., 2009), and these empirical results suggest that vitamin B12 availability could also have an important interactive role in modulating such stoichiometric shifts in the future high-CO2 ocean.
Fe requirements and NUEs
Cells grown under B12-replete/high-pCO2 conditions had lower Fe quotas than B12-replete/low-pCO2 treatments (Figure 3a). The negative correlation between Fe requirements and pCO2 could be a reflection of the lower external availability of Fe at high pCO2—reduced uptake rates of Fe bound to model carboxylic acid and hydroxamate-type organic ligands were recently reported in diatom cultures at high pCO2/low pH (Shi et al., 2010). In our experiments, Fe was buffered by EDTA (via carboxylic acid moieties), and the lower observed Fe:P in B12-replete cultures at 670 p.p.m. pCO2 (Figure 3a) might be a result of lower Fe availability. However, Fe:P of B12-limited/670 p.p.m. pCO2-grown cells was the highest of all CO2 treatments (Figure 3a), suggesting that another mechanism, in this case perhaps the influence of CO2 on cellular Fe requirements, might have a larger effect than the reduction in Fe bioavailability.
A reduced Fe requirement for photosynthesis and respiration-generated energy to power carbonic anhydrases (CAs) or other carbon-concentrating mechanism-related cellular machinery could be reason for lower Fe:P (Figure 3a) and higher FeNUE (Figure 4a) of B12-replete/670 p.p.m. pCO2 cells. Theoretical calculations predict that cells saturated with CO2 require less ATP and thus approximately one-third less Fe in the photosynthetic and respiratory electron transport chains (Raven, 1991). It is notable that in B12-replete experiments, there is a counterintuitive relationship between Fe:P and physiological measurements—growth rate and primary productivity increase with pCO2, whereas Fe requirements simultaneously decrease. Further studies assessing the relative importance of reduced in situ Fe bioavailability (Shi et al., 2010) and lower cellular Fe requirements (this study) is warranted.
The synergistic relationship between vitamin B12 availability and intracellular Fe quotas is not totally unexpected. Both Fe (Strzepek and Harrison, 2004) and phylloquinone (vitamin K1; a macromolecule that requires B12-dependent S-adenosyl methinone for its synthesis; Lohmann et al., 2006) serve as electron carriers in photosystem I. Our empirical lab results (Figure 3a) suggest that Fe requirements of Attheya are lower (and FeNUE higher) when B12 was replete at 370 and 670 p.p.m. pCO2, perhaps because photosynthetic electron transfer is more dependent on phylloquinone. In contrast, under B12-limited conditions, the Fe requirement of the organism was higher (and FeNUE lower), suggesting that electron transfer could be more dependent on Fe –complexes, such as the cytochrome and ferredoxin. Although future studies will be needed to test the validity of this hypothesis, the ‘photosynthetic architecture’ of diatoms has been shown to depend on Fe availability (Strzepek and Harrison, 2004). The dominant electron transfer mechanism in photosystem I could also vary with ambient B12 supply.
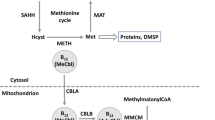
There are likely other indirect biochemical relationships between B12/CO2 availability and Fe requirements, as B12, CO2 and Fe are each intimately involved in multiple fundamental biochemical pathways, such as photosynthesis (see above), nutrient acquisition (for example, involvement of Fe in nitrate assimilation; Milligan and Harrison, 2000) and amino-acid synthesis (for example, B12-dependent methionine synthase, metH; Croft et al., 2005). Because B12 is required for key enzymes, such as metH and methylmalonyl CoA mutase (Rodionov et al., 2003; Croft et al., 2005), Fe requirements may be affected downstream of where these enzymes are located in metabolic pathways. The role of B12 in methionine synthesis could affect cell-wide processes because of the need of methionine for biosynthesis of proteins and various critical transmethylation reactions via S-adenosyl methinone (Fontecave et al., 2004).
Co requirements and NUEs
Under B12-replete conditions, Co:P ratios were ∼5- to 14-fold higher in comparison with B12-limited cells (Figure 3b), suggesting that Co-containing vitamin B12 could comprise a significant fraction of the total cellular Co quota, and/or that B12 limitation could result in lower Co requirements. In B12-limited experiments, we calculated the percent of cellular Co that likely originates from assimilated B12, assuming that the B12 added to the media was completely bioavailable. If B12 was fully utilized, Co from B12 (one Co atom per B12 molecule) accounted for ∼78 to >100% of measured mean cellular Co (Table 2). When B12 was limiting, CoNUE at 200 and 370 p.p.m. pCO2 were relatively high because of the substantially lower Co quotas (Figures 3b and 4b). We are unable to make a similar Co mass balance calculation for the B12-replete cells, as our B12-replete media had 370 pM Co–B12 and 50 nM Co, which were both in excess of the measured cellular Co concentrations (62–133 pM; Table 2). In addition to Co in vitamin B12, Co has also been identified as a metabolic substitute in diatoms for Zn in CA (Morel et al., 1994; Sunda and Huntsman, 1995b).
Zn and Cd requirements and use efficiencies
When Attheya was grown under B12-replete/high pCO2, Zn:P and Cd:P ratios were low and ZnNUE and CdNUE were high (Figures 3c, d, 4c and d). Lower Zn:P is consistent with higher CO2 availability and a decreased Zn requirement for Zn-containing CA used for catalyzing the conversion of HCO3− to CO2 (Morel et al., 1994; Lane and Morel, 2000a). Previous laboratory culture experiments with the diatoms Thalassiosira weissflogii and Thalassiosira pseudonana have also found higher Zn quotas of cells grown at lower pCO2 in comparison with cells grown at present-day pCO2 (Lane and Morel, 2000a; Sunda and Huntsman, 2005). Lower Cd:P ratios at high pCO2 in B12-replete treatments could be the result of the downregulation of a Cd-specific CA (Lane and Morel, 2000b; Lane et al., 2005; Shi et al., 2010).
In addition to CO2, vitamin B12 availability might also affect Zn-based CAs. Methionine, among two other amino acids, was found to be a critical component of a hydrophobic cluster on the C-terminal α-helix that predicated formation, and possibly function, of the β-type Zn-containing CA (PtCA1) of the diatom Phaeodactylum tricornutum (Kitao and Matsuda, 2009).
Ecological implications
Extrapolation from culture-based studies to natural ecosystems requires caution, but these results could have implications for altered (co)limitation patterns of phytoplankton in a changing ocean. At present-day pCO2 (370 p.p.m.) and estimated pCO2 before year 2100 (670 p.p.m.), B12 limitation resulted in higher Fe quotas and lower NUE of Fe, Zn and Cd. Although diatoms in field experiments in the subarctic Pacific and Southern Oceans have been shown to be limited by Fe and/or B12 (Bertrand et al., 2007; Koch et al., submitted), our experimental results imply that the consequences of low B12 availability at current and future pCO2 are a higher Fe requirement, lower growth/productivity and thus lower FeNUE. B12 limitation of diatoms like Attheya could drive the subarctic Pacific and Southern Oceans (that are already relatively low in Fe) further toward B12/Fe colimiting HNLC conditions. In contrast, if B12 availability increases in the future ocean, a lower Fe requirement (and higher Fe/Zn/CdNUE) by diatoms might result in community shifts toward this group, a decoupling of their growth from Fe availability and strengthening of the biological C pump in these regimes.
In addition, Attheya belongs to a functional group of chain-forming centric diatoms that have been observed to proliferate in Fe addition bottle experiments and mesoscale Fe fertilization experiments in the HNLC regimes of the subarctic North Pacific, Southern Ocean and coastal California upwelling (Martin et al., 1989; Hutchins and Bruland, 1998; de Baar et al., 2005), and are believed to form resting spores that contribute to C export in the coastal subarctic and northeast Pacific (Grimm et al., 1996; Saino et al., 1998). Based on our experimental results, without an adequate supply of B12, and despite a high availability of Fe, Chaetoceros-like diatoms would have a similar Fe requirement but use Fe much less efficiently to achieve only about half the growth and carbon fixation rates.
The present study highlights the linkages between organic growth factor limitation, trace metal requirements and NUE, and phytoplankton productivity under varying availabilities of pCO2. For example, our results under conditions of the last glacial maximum suggest that increases in eolian Fe deposition alone to surface waters of HNLC areas during that period may not have been sufficient to reduce atmospheric CO2 via the biological pump, contrary to Martin's ‘iron hypothesis’ (Martin, 1990). Our results showed that high primary productivity was only attained when B12 was available. The relevance of Fe availability to the biological drawdown of atmospheric CO2 during glacial periods has also recently been questioned by Boyd et al. (2010). Further work toward elucidating these types of interactions is needed to understand the biogeochemical consequences of natural and anthropogenic Fe fertilization in HNLC regimes, and the potential impacts of changing pCO2 on global C cycling in the present-day and future ocean.
References
Behrenfeld MJ, Falkowski PG . (1997). Photosynthetic rates derived from satellite-based chlorophyll concentration. Limnol Oceanogr 42: 1–20.
Betrand EM, Saito MA, Rose JM, Riesselman CR, Lohan MC, Noble AE et al. (2007). Vitamin B12 and iron colimitation of phytoplankton growth in the Ross Sea. Limnol Oceanogr 52: 1079–1093.
Boyd PW, Mackie DS, Hunter KA . (2010). Aerosol iron deposition to the surface ocean-modes of iron supply and biological responses. Mar Chem 120: 128–143.
Carlucci AF, Silbernagel SB . (1969). Effect of vitamin concentrations on growth and development of vitamin-requiring algae. J Phycol 5: 64–67.
Crawford RM, Hinz F, Koschinski P . (2000). The combination of Chaetoceros gaussii (Bacillariophyta) with Attheya. Phycologia 39: 238–244.
Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, Smith AG . (2005). Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438: 90–93.
de Baar HJW, Boyd PW, Coale KH, Landry MR, Tsuda A, Assmy P et al. (2005). Synthesis of iron fertilization experiments: from the iron age in the age of enlightenment. J Geophys Res—Oceans 110: C09S16.
Dickson AG, Goyet C . (1994). Handbook of Methods for the Analysis of the Various Parameters of the Carbon Dioxide System in Sea Water (Ver. 2). Department of Energy, ORNL/CDIAC-74: Oak Ridge, Tenn.
Droop MR . (1957). Vitamin B12 in marine ecology. Nature 180: 1041–1042.
Droop MR . (2007). Vitamins, phytoplankton and bacteria: symbiosis or scavenging? J Plankton Res 29: 107–113.
Eggimann DW, Betzer PR . (1976). Decomposition and analysis of refractory oceanic suspended materials. Anal Chem 48: 886–890.
Fontecave M, Atta M, Mulliez E . (2004). S-adenosylmethionine: nothing goes to waste. Trends Biochem Sci 29: 243–249.
Fu FX, Warner ME, Zhang Y, Feng Y, Hutchins DA . (2007). Effects of increased temperature and CO2 on photosynthesis, growth and elemental ratios of marine Synechococcus and Prochlorococcus (Cyanobacteria). J Phycol 43: 485–496.
Fu FX, Zhang Y, Leblanc K, Sañudo-Wilhelmy SA, Hutchins DA . (2005). The biological and biogeochemical consequences of phosphate scavenging onto phytoplankton cell surfaces. Limnol Oceanogr 50: 1459–1472.
Gobler CJ, Norman C, Panzeca C, Taylor GT, Sañudo-Wilhelmy SA . (2007). Effect of B-vitamins (B1, B12) and inorganic nutrients on algal bloom dynamics in a coastal ecosystem. Aquat Microb Ecol 49: 181–194.
Grimm KA, Lange CB, Gill AS . (1996). Biological forcing of hemipelagic sedimentary laminae: evidence from ODP Site 893, Santa Barbara Basin, California. J Sed Res 66: 613–624.
Hutchins DA, Bruland KW . (1998). Iron-limited diatom growth and Si:N uptake ratios in a coastal upwelling regime. Nature 393: 561–564.
Hutchins DA, Mulholland MR, Fu FX . (2009). Nutrient cycles and marine microbes in a CO2-enriched ocean. Oceanography 22: 128–145.
Kitao Y, Matsuda Y . (2009). Formation of macromolecular complexes of carbonic anhydrases in the chloroplast of a marine diatom by the action of the C-terminal helix. Biochem J 419: 681–688.
Koch F, Marcoval A, Panzeca C, Bruland KW, Sañudo-Wilhelmy SA, Gobler CJ . (submitted). The effect of vitamin B12, nitrogen and iron on phytoplankton growth and community structure in the Gulf of Alaska.
Lane TW, Morel FMM . (2000a). Regulation of carbonic anhydrase expression by zinc, cobalt, and carbon dioxide in the marine diatom Thalassiosira weissflogii. Plant Physiol 123: 345–352.
Lane TW, Morel FMM . (2000b). A biological function for cadmium in marine diatoms. Proc Natl Acad Sci USA 97: 4627–4631.
Lane TW, Saito MA, George GN, Pickering IJ, Prince RC, Morel FMM . (2005). A cadmium enzyme from a marine diatom. Nature 435: 42.
Leblanc K, Hare CE, Boyd PW, Bruland KW, Sohst B, Pickmere S et al. (2005). Fe and Zn effects on the Si cycle and diatom community structure in two contrasting high and low-silicate HNLC areas. Deep-Sea Res I 52: 1842–1864.
Lohmann A, Schöttler MA, Bréhélin C, Kessler F, Bock R, Cahoon EB et al. (2006). Deficiency in phylloquinone (vitamin K1) methylation affects prenyl quinone distribution, photosystem I abundance, and anthocyanin accumulation in the Arabidopsis AtmenG mutant. J Biol Chem 281: 40461–40472.
Marchetti A, Parker MS, Moccia LP, Lin EO, Arrieta AL, Ribalet F et al. (2009). Ferritin is used for iron storage in bloom-forming marine pinnate diatoms. Nature 457: 467–470.
Martin JH . (1990). Glacial-interglacial CO2 change: the iron hypothesis. Paleoceanography 5: 1–13.
Martin JH, Gordon RM, Fitzwater S, Broenkow WW . (1989). Vertex: phytoplankton iron studies in the Gulf of Alaska. Deep Sea Res Part A 36: 649–680.
Milligan AJ, Harrison PJ . (2000). Effects of non-steady-state iron limitation on nitrogen assimilation enzymes in the marine diatom Thalassiosira weissflogii (Bacillariophyceae). J of Phycol 36: 78–86.
Morel FMM, Reinfelder JR, Roberts SB, Chamberlain CP, Lee JG, Yee D . (1994). Zinc and carbon co-limitation of marine-phytoplankton. Nature 369: 740–742.
Panzeca C, Beck AJ, Tovar-Sanchez A, Segovia-Zavala J, Taylor GT, Gobler CJ et al. (2009). Distributions of dissolved vitamin B12 and Co in coastal and open-ocean environments. Estuar Coast Shelf Sci 85: 223–230.
Panzeca C, Tovar-Sanchez A, Agusti S, Reche I, Duarte CM, Taylor GT . (2006). B vitamin as regulators of phytoplankton dynamics. EOS Trans AGU 87: 593–596.
Petit JR, Jouzel J, Raynaud D, Barkov NI, Barnola JM, Basile I et al. (1999). Climate and atmospheric history of the past 420 000 years from the Vostok ice core, Antarctica. Nature 399: 429–436.
Price NM, Harrison GI, Hering JG, Hudson RJM, Nirel PMV, Palenik B et al. (1988/89). Preparation and chemistry of the artificial algal culture medium Aquil. Biol Oceanogr 6: 443–461.
Provasoli L, Carlucci AF . (1974). Vitamins and growth regulators. In: Stewart WDP, Abbott MR (eds). Algal Physiology and Biochemistry. Blackwell Science: Malden, Mass, pp 741–787.
Raven JA . (1991). Physiology of inorganic C acquisition and implications for resource use efficiency by marine phytoplankton: relation to increased CO2 and temperature. Plant Cell Environ 14: 779–794.
Riebesell U, Schulz KG, Bellerby RG, Botros M, Fritsche P, Meyerhöfer M et al. (2007). Enhanced biological carbon consumption in a high CO2 ocean. Nature 450: 545–548.
Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS . (2003). Comparative genomics of the vitamin B12 metabolism and regulation in prokaryotes. J Biol Chem 278: 41148–41159.
Saino T, Shang S, Mino Y, Suzuki K, Nomura H, Saitoh S et al. (1998). Short term variability of particle fluxes and its relation to variability in sea surface temperature and chlorophyll a field detected by ocean color and temperature scanner (OCTS) off Sanriku, northwestern north Pacific in the spring of 1997. J Oceanogr 54: 583–592.
Sañudo-Wilhelmy SA, Gobler CJ, Okbamichael M, Taylor GT . (2006). Regulation of phytoplankton dynamics by vitamin B12 . Geophys Res Lett 33: L04604.
Sañudo-Wilhelmy SA, Kustka AB, Gobler CJ, Hutchins DA, Yang M, Lwiza K et al. (2001). Phosphorus limitation of nitrogen fixation by Trichodesmium in the central Atlantic Ocean. Nature 411: 66–69.
Shi DL, Xu Y, Hopkinson BM, Morel FMM . (2010). Effect of ocean acidification on iron availability to marine phytoplankton. Science 327: 676–679.
Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB et al. (2007). Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press: Cambridge, United Kingdom and New York, NY.
Strzepek RF, Harrison PJ . (2004). Photosynthetic architecture differs in coastal and oceanic diatoms. Nature 431: 689–692.
Sunda WG, Huntsman SA . (1995a). Iron uptake and growth limitation in oceanic and coastal phytoplankton. Mar Chem 50: 189–206.
Sunda WG, Huntsman SA . (1995b). Cobalt and zinc interreplacement in marine phytoplankton: Biological and geochemical implications. Limnol Oceanogr 40: 1404–1417.
Sunda WG, Huntsman SA . (2005). Effect of CO2 supply and demand on zinc uptake and growth limitation in a coastal diatom. Limnol Oceanogr 50: 1181–1192.
Swift DG, Taylor WR . (1974). Growth of vitamin B12-limited cultures Thalassiosira pseudonana, Monochrysis lutheri, and Isochrysis galbana. J Phycol 10: 385–391.
Tang YZ, Koch F, Gobler CJ . (2010). Most harmful algal bloom species are vitamin B1 and B12 auxotrophs. Proc Natl Acad Sci USA 107: 20756–20761.
Tovar-Sanchez A, Sañudo-Wilhelmy SA, Garcia-Vargas M, Weaver RS, Popels LC, Hutchins DA . (2003). A trace metal clean reagent to remove surface-bound iron from marine phytoplankton. Mar Chem 82: 91–99.
Acknowledgements
This study was supported by the US National Science Foundation grants OCE 0962209 to SAS, OCE 0850730 to FXF, and OCE 0722337 and 0825319 to DAH.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
King, A., Sañudo-Wilhelmy, S., Leblanc, K. et al. CO2 and vitamin B12 interactions determine bioactive trace metal requirements of a subarctic Pacific diatom. ISME J 5, 1388–1396 (2011). https://doi.org/10.1038/ismej.2010.211
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2010.211
Keywords
This article is cited by
-
Roles and sources of B vitamins in the marine ecosystem
Reviews in Fish Biology and Fisheries (2024)
-
Dynamics and geochemical responses of dissolved metals (Mn and Cu) in a subtropical estuary, China
Environmental Science and Pollution Research (2023)
-
Availability of vitamin B12 and its lower ligand intermediate α-ribazole impact prokaryotic and protist communities in oceanic systems
The ISME Journal (2022)
-
Effects of elevated pCO2 on the photosynthetic performance of the sea ice diatoms Navicula directa and Navicula glaciei
Journal of Applied Phycology (2022)
-
Short-term response of pelagic planktonic communities after inoculation with the mass cultured dinoflagellate Alexandrium affine in a large-scale mesocosm experiment
Journal of Applied Phycology (2021)