Abstract
Background/Objectives:
We investigated the effect of long-term treatment with lobeglitazone, a novel thiazolidinedione-based activator of peroxisome proliferator-activated receptor gamma, on adipose tissue (AT), focusing on its effects on insulin resistance in obese db/db mice.
Methods:
Seven-week-old male db/db mice were assigned to either a vehicle-treated (n=8) or lobeglitazone-treated (n=8) group. Lobeglitazone (1 mg kg−1 daily) was injected intraperitoneally for 20 weeks.
Results:
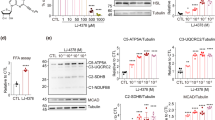
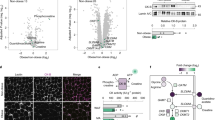
Lobeglitazone treatment for 20 weeks resulted in a remarkably improved glycemic index, including significantly decreased glucose levels, enhanced insulin sensitivity and preserved pancreatic beta cells. Both whole body and subcutaneous AT weight increased in the lobeglitazone-treated group. However, lobeglitazone induced an increase in the number of small adipocyte in both epididymal and subcutaneous AT, with a significant weight decrease in the epididymal AT of db/db mice. Using flow cytometry, the CD11c-positive M1 macrophages and CD206-positive M2 macrophages in the epididymal AT were observed to exhibit a decreased M1-to-M2 ratio in lobeglitazone-treated db/db mice. Furthermore, in the lobeglitazone-treated group, interscapular brown AT was clearly visualized by 18F-fluoro-2-deoxy-D-glucose positron emission tomography combined with computed tomography (18F-FDG-PET/CT) and its mass was significantly greater than that of the vehicle-treated group. In the lobeglitazone-treated group, beige-specific gene expression and the number of mitochondria in white AT were upregulated. Lobeglitazone, with upregulating interferon regulatory factor-4 (a key transcriptional regulator of thermogenesis), promoted the development of brown adipocytes and the differentiation of white adipocytes into beige adipocytes.
Conclusions:
Long-term lobeglitazone treatment has a beneficial role in remodeling and ameliorating inflammation in white AT and in glycemic control, in relation to insulin sensitivity in obese db/db mice. Moreover, lobeglitazone induced the differentiation of brown and beige adipocytes. Collectively, our data suggest that lobeglitazone treatment provides promising effects on white and brown AT as well as great improvement in glycemic control, as a potent insulin sensitizer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Zimmet PZ, Magliano DJ, Herman WH, Shaw JE . Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol 2014; 2: 56–64.
Kahn SE . The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 2003; 46: 3–19.
Wild S, Roglic G, Green A, Sicree R, King H . Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047–1053.
Zimmet P, Alberti KG, Shaw J . Global and societal implications of the diabetes epidemic. Nature 2001; 414: 782–787.
McKeigue PM, Shah B, Marmot MG . Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet 1991; 337: 382–386.
Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Jama 2003; 289: 76–79.
Lebovitz HE, Banerji MA . Insulin resistance and its treatment by thiazolidinediones. Recent Prog Horm Res 2001; 56: 265–294.
Spiegelman BM . PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes 1998; 47: 507–514.
Miyazaki Y, Glass L, Triplitt C, Matsuda M, Cusi K, Mahankali A et al. Effect of rosiglitazone on glucose and non-esterified fatty acid metabolism in Type II diabetic patients. Diabetologia 2001; 44: 2210–2219.
Sugii S, Olson P, Sears DD, Saberi M, Atkins AR, Barish GD et al. PPARgamma activation in adipocytes is sufficient for systemic insulin sensitization. Proc Natl Acad Sci USA 2009; 106: 22504–22509.
Koppen A, Kalkhoven E . Brown vs white adipocytes: the PPARgamma coregulator story. FEBS Lett 2010; 584: 3250–3259.
de Souza CJ, Eckhardt M, Gagen K, Dong M, Chen W, Laurent D et al. Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes 2001; 50: 1863–1871.
Koppaka S, Kehlenbrink S, Carey M, Li W, Sanchez E, Lee DE et al. Reduced adipose tissue macrophage content is associated with improved insulin sensitivity in thiazolidinedione-treated diabetic humans. Diabetes 2013; 62: 1843–1854.
Koh YJ, Park BH, Park JH, Han J, Lee IK, Park JW et al. Activation of PPAR gamma induces profound multilocularization of adipocytes in adult mouse white adipose tissues. Exp Mol Med 2009; 41: 880–895.
Kim SH, Plutzky J . Brown fat and browning for the treatment of obesity and related metabolic disorders. Diabetes Metab J 2016; 40: 12–21.
Kajimura S, Saito M . A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu Rev Physiol 2014; 76: 225–249.
Saito M . Brown adipose tissue as a regulator of energy expenditure and body fat in humans. Diabetes Metab J 2013; 37: 22–29.
Hall GC, Smith HT, Curtis B, McMahon AD . Changes and predictors for change to thiazolidinedione prescribing in UK primary care following the rosiglitazone safety warning. Int J Clin Pract 2011; 65: 586–591.
Hampp C, Pippins J . Pioglitazone and bladder cancer: FDA's assessment. Pharmacoepidemiol Drug Safety 2017; 26: 117–118.
Jin SM, Park CY, Cho YM, Ku BJ, Ahn CW, Cha BS et al. Lobeglitazone and pioglitazone as add-ons to metformin for patients with type 2 diabetes: a 24-week, multicentre, randomized, double-blind, parallel-group, active-controlled, phase III clinical trial with a 28-week extension. Diabetes Obes Metab 2015; 17: 599–602.
Kim SH, Kim SG, Kim DM, Woo JT, Jang HC, Chung CH et al. Safety and efficacy of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 52 weeks: An open-label extension study. Diabetes Res Clin Pract 2015; 110: e27–e30.
Lee YH, Kim JH, Kim SR, Jin HY, Rhee EJ, Cho YM et al. Lobeglitazone, a novel thiazolidinedione, improves non-alcoholic fatty liver disease in type 2 diabetes: its efficacy and predictive factors related to responsiveness. J Korean Med Sci 2017; 32: 60–69.
Lim S, Lee KS, Lee JE, Park HS, Kim KM, Moon JH et al. Effect of a new PPAR-gamma agonist, lobeglitazone, on neointimal formation after balloon injury in rats and the development of atherosclerosis. Atherosclerosis 2015; 243: 107–119.
Wang X, Minze LJ, Shi ZZ . Functional imaging of brown fat in mice with 18F-FDG micro-PET/CT. J Vis Exp 2012; 69: e4060.
Zhang Z, Zhang H, Li B, Meng X, Wang J, Zhang Y et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat commun 2014; 5: 5493.
Ohno H, Shinoda K, Ohyama K, Sharp LZ, Kajimura S . EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature 2013; 504: 163–167.
Kraynik SM, Miyaoka RS, Beavo JA . PDE3 and PDE4 isozyme-selective inhibitors are both required for synergistic activation of brown adipose tissue. Mol Pharmacol 2013; 83: 1155–1165.
Lee YH, Kim SH, Lee YJ, Kang ES, Lee BW, Cha BS et al. Transcription factor Snail is a novel regulator of adipocyte differentiation via inhibiting the expression of peroxisome proliferator-activated receptor gamma. Cell Mol Life Sci 2013; 70: 3959–3971.
Jung HS, Chung KW, Won Kim J, Kim J, Komatsu M, Tanaka K et al. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab 2008; 8: 318–324.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Galarraga M, Campion J, Munoz-Barrutia A, Boque N, Moreno H, Martinez JA et al. Adiposoft: automated software for the analysis of white adipose tissue cellularity in histological sections. J Lipid Res 2012; 53: 2791–2796.
Zembruski NC, Stache V, Haefeli WE, Weiss J . 7-Aminoactinomycin D for apoptosis staining in flow cytometry. Anal Biochem 2012; 429: 79–81.
Song YM, Lee YH, Kim JW, Ham DS, Kang ES, Cha BS et al. Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway. Autophagy 2015; 11: 46–59.
Shinoda K, Ohyama K, Hasegawa Y, Chang HY, Ogura M, Sato A et al. Phosphoproteomics identifies CK2 as a negative regulator of beige adipocyte thermogenesis and energy expenditure. Cell Metab 2015; 22: 997–1008.
Enerback S . Casein kinase 2—a kinase that inhibits brown fat formation. Cell Metab 2015; 22: 958–959.
Katahira H, Nagamatsu S, Ozawa S, Nakamichi Y, Yamaguchi S, Furukawa H et al. Acute inhibition of proinsulin biosynthesis at the translational level by palmitic acid. Biochem Biophys Res Commun 2001; 282: 507–510.
Kanda Y, Shimoda M, Hamamoto S, Tawaramoto K, Kawasaki F, Hashiramoto M et al. Molecular mechanism by which pioglitazone preserves pancreatic beta-cells in obese diabetic mice: evidence for acute and chronic actions as a PPARgamma agonist. Am J Physiol Endocrinol Metab 2010; 298: E278–E286.
Bell DS . Beta-cell rejuvenation with thiazolidinediones. Am J Med 2003; 115 (Suppl 8A): 20s–23s.
Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA . An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem 1995; 270: 12953–12956.
Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ . Abdominal fat and insulin resistance in normal and overweight women: direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes 1996; 45: 633–638.
Park J, Lee ES, Lee DY, Kim J, Park SE, Park CY et al. Waist circumference as a marker of obesity is more predictive of coronary artery calcification than body mass index in apparently healthy Korean adults: the Kangbuk Samsung Health Study. Endocrinol Metab 2016; 31: 559–566.
Arner P . Differences in lipolysis between human subcutaneous and omental adipose tissues. Ann Med 1995; 27: 435–438.
Rattarasarn C, Leelawattana R, Soonthornpun S, Setasuban W, Thamprasit A, Lim A et al. Regional abdominal fat distribution in lean and obese Thai type 2 diabetic women: relationships with insulin sensitivity and cardiovascular risk factors. Metab Clin Exp 2003; 52: 1444–1447.
Ibrahim MM . Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 2010; 11: 11–18.
Akazawa S, Sun F, Ito M, Kawasaki E, Eguchi K . Efficacy of troglitazone on body fat distribution in type 2 diabetes. Diabetes care 2000; 23: 1067–1071.
Lumeng CN, Bodzin JL, Saltiel AR . Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007; 117: 175–184.
Wellen KE, Hotamisligil GS . Obesity-induced inflammatory changes in adipose tissue. J Clin Invest 2003; 112: 1785–1788.
Sun K, Kusminski CM, Scherer PE . Adipose tissue remodeling and obesity. J Clin Invest 2011; 121: 2094–2101.
Spencer M, Unal R, Zhu B, Rasouli N, McGehee RE Jr, Peterson CA et al. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab 2011; 96: E1990–E1998.
Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol 2009; 29: 1575–1591.
Harms M, Seale P . Brown and beige fat: development, function and therapeutic potential. Nat Med 2013; 19: 1252–1263.
Hibi M, Oishi S, Matsushita M, Yoneshiro T, Yamaguchi T, Usui C et al. Brown adipose tissue is involved in diet-induced thermogenesis and whole-body fat utilization in healthy humans. Int J Obes 2016; 40: 1655–1661.
Wang Q, Zhang M, Xu M, Gu W, Xi Y, Qi L et al. Brown adipose tissue activation is inversely related to central obesity and metabolic parameters in adult human. PloS One 2015; 10: e0123795.
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360: 1509–1517.
Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009; 360: 1518–1525.
Inagaki T, Sakai J, Kajimura S . Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat Rev Mol Cell Biol 2016; 17: 480–495.
Ohno H, Shinoda K, Spiegelman BM, Kajimura S . PPARgamma agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 2012; 15: 395–404.
Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J et al. Transcriptional control of adipose lipid handling by IRF4. Cell Metab 2011; 13: 249–259.
Eguchi J, Kong X, Tenta M, Wang X, Kang S, Rosen ED . Interferon regulatory factor 4 regulates obesity-induced inflammation through regulation of adipose tissue macrophage polarization. Diabetes 2013; 62: 3394–3403.
Kong X, Banks A, Liu T, Kazak L, Rao RR, Cohen P et al. IRF4 is a key thermogenic transcriptional partner of PGC-1alpha. Cell 2014; 158: 69–83.
Acknowledgements
We would like to thank Professor Shingo Kajimura, University of California, San Francisco for kindly providing us with the immortalized brown adipocyte line. We are grateful to Dr Seok Jong Chung, Department of Neurology, Yonsei University College of Medicine for his help with analyses of adipocyte size measurement. This study was supported by research grant from the Yonsei University Industry-Academic Cooperation Foundation (2014-31-0728, BSC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Kim, G., Lee, Yh., Yun, M. et al. Effects of lobeglitazone, a novel thiazolidinedione, on adipose tissue remodeling and brown and beige adipose tissue development in db/db mice. Int J Obes 42, 542–551 (2018). https://doi.org/10.1038/ijo.2017.222
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2017.222