Abstract
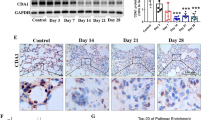
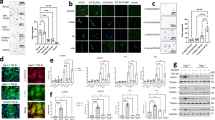
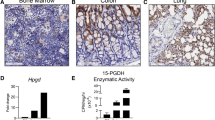
Idiopathic pulmonary fibrosis (IPF) is a fatal disease with a median survival of 3–4 years after diagnosis. It is the most frequent form of a group of interstitial pneumonias of unknown etiology. Current available therapies prevent deterioration of lung function but no therapy has shown to improve survival. Periostin is a matricellular protein of the fasciclin 1 family. There is increased deposition of periostin in lung fibrotic tissues. Here we evaluated whether small interfering RNA or antisense oligonucleotide against periostin inhibits lung fibrosis by direct administration into the lung by intranasal route. Pulmonary fibrosis was induced with bleomycin and RNA therapeutics was administered during both acute and chronic phases of the disease. The levels of periostin and transforming growth factor-β1 in airway fluid and lung tissue, the deposition of collagen in lung tissue and the lung fibrosis score were significantly reduced in mice treated with siRNA and antisense against periostin compared to control mice. These findings suggest that direct administration of siRNA or antisense oligonucleotides against periostin into the lungs is a promising alternative therapeutic approach for the management of pulmonary fibrosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Wynn TA . Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 2007; 117: 524–529.
Gross TJ, Hunninghake GW . Idiopathic pulmonary fibrosis. N Engl J Med 2001; 345: 517–525.
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824.
George G, Vaid U, Summer R . Therapeutic advances in idiopathic pulmonary fibrosis. Clin Pharmacol Ther 2016; 99: 30–32.
Todd NW, Luzina IG, Atamas SP . Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair 2012; 5: 11.
Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res 1999; 14: 1239–1249.
Izuhara K, Conway SJ, Moore BB, Matsumoto H, Holweg CT, Matthews JG et al. Roles of periostin in respiratory disorders. Am J Respir Crit Care Med 2016; 193: 949–956.
Conway SJ, Doetschman T, Azhar M . The inter-relationship of periostin, TGF beta, and BMP in heart valve development and valvular heart diseases. ScientificWorldJournal 2011; 11: 1509–1524.
Naik PK, Bozyk PD, Bentley JK, Popova AP, Birch CM, Wilke CA et al. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2012; 303: L1046–L1056.
Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L et al. Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA 2010; 107: 14170–14175.
Uchida M, Shiraishi H, Ohta S, Arima K, Taniguchi K, Suzuki S et al. Periostin, a matricellular protein, plays a role in the induction of chemokines in pulmonary fibrosis. Am J Respir Cell Mol Biol 2012; 46: 677–686.
Chery J, Naar A . RNA therapeutics: RNAi and antisense mechanisms and clinical applications. Postdoc J 2016; 4: 35–50.
Carthew RW, Sontheimer EJ . Origins and mechanisms of miRNAs and siRNAs. Cell 2009; 136: 642–655.
Novina CD, Sharp PA . The RNAi revolution. Nature 2004; 430: 161–164.
Geary RS, Norris D, Yu R, Bennett CF . Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev 2015; 87: 46–51.
Crooke ST, Geary RS . Clinical pharmacological properties of mipomersen (Kynamro), a second generation antisense inhibitor of apolipoprotein B. Br J Clin Pharmacol 2013; 76: 269–276.
Moreno PM, Pego AP . Therapeutic antisense oligonucleotides against cancer: hurdling to the clinic. Front Chem 2014; 2: 87.
Ozcan G, Ozpolat B, Coleman RL, Sood AK, Lopez-Berestein G . Preclinical and clinical development of siRNA-based therapeutics. Adv Drug Deliv Rev 2015; 87: 108–119.
Ashcroft T, Simpson JM, Timbrell V . Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 1988; 41: 467–470.
Wolters PJ, Collard HR, Jones KD . Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol 2014; 9: 157–179.
Tajiri M, Okamoto M, Fujimoto K, Johkoh T, Ono J, Tominaga M et al. Serum level of periostin can predict long-term outcome of idiopathic pulmonary fibrosis. Respir Investig 2015; 53: 73–81.
D'Alessandro-Gabazza CN, Kobayashi T, Boveda-Ruiz D, Takagi T, Toda M, Gil-Bernabe P et al. Development and preclinical efficacy of novel transforming growth factor-beta1 short interfering RNAs for pulmonary fibrosis. Am J Respir Cell Mol Biol 2012; 46: 397–406.
Ohta S, Okamoto M, Fujimoto K, Sakamoto N, Takahashi K, Yamamoto H et al. The usefulness of monomeric periostin as a biomarker for idiopathic pulmonary fibrosis. PLoS ONE 2017; 12: e0174547.
Moschos SA, Spinks K, Williams AE, Lindsay MA . Targeting the lung using siRNA and antisense based oligonucleotides. Curr Pharm Des 2008; 14: 3620–3627.
Snove O Jr ., Holen T . Many commonly used siRNAs risk off-target activity. Biochem Biophys Res Commun 2004; 319: 256–263.
Behlke MA . Progress towards in vivo use of siRNAs. Mol Ther 2006; 13: 644–670.
Nyce JW, Metzger WJ . DNA antisense therapy for asthma in an animal model. Nature 1997; 385: 721–725.
Bitko V, Musiyenko A, Shulyayeva O, Barik S . Inhibition of respiratory viruses by nasally administered siRNA. Nat Med 2005; 11: 50–55.
Finotto S, De Sanctis GT, Lehr HA, Herz U, Buerke M, Schipp M et al. Treatment of allergic airway inflammation and hyperresponsiveness by antisense-induced local blockade of GATA-3 expression. J Exp Med 2001; 193: 1247–1260.
Torres R, Herrerias A, Serra-Pages M, Roca-Ferrer J, Pujols L, Marco A et al. An intranasal selective antisense oligonucleotide impairs lung cyclooxygenase-2 production and improves inflammation, but worsens airway function, in house dust mite sensitive mice. Respir Res 2008; 9: 72.
Miller MA, Stabenow JM, Parvathareddy J, Wodowski AJ, Fabrizio TP, Bina XR et al. Visualization of murine intranasal dosing efficiency using luminescent Francisella tularensis: effect of instillation volume and form of anesthesia. PLoS One 2012; 7: e31359.
Wynn TA . Cellular and molecular mechanisms of fibrosis. J Pathol 2008; 214: 199–210.
Bagnato G, Harari S . Cellular interactions in the pathogenesis of interstitial lung diseases. Eur Respir Rev 2015; 24: 102–114.
Luzina IG, Todd NW, Iacono AT, Atamas SP . Roles of T lymphocytes in pulmonary fibrosis. J Leukoc Biol 2008; 83: 237–244.
Stahl M, Schupp J, Jager B, Schmid M, Zissel G, Muller-Quernheim J et al. Lung collagens perpetuate pulmonary fibrosis via CD204 and M2 macrophage activation. PLoS ONE 2013; 8: e81382.
Moore BB, Hogaboam CM . Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2008; 294: L152–L160.
Leask A, Abraham DJ . TGF-beta signaling and the fibrotic response. FASEB J 2004; 18: 816–827.
Crawford J, Nygard K, Gan BS, O'Gorman DB . Periostin induces fibroblast proliferation and myofibroblast persistence in hypertrophic scarring. Exp Dermatol 2015; 24: 120–126.
Yang L, Serada S, Fujimoto M, Terao M, Kotobuki Y, Kitaba S et al. Periostin facilitates skin sclerosis via PI3K/Akt dependent mechanism in a mouse model of scleroderma. PLoS ONE 2012; 7: e41994.
Urawa M, Kobayashi T, D'Alessandro-Gabazza CN, Fujimoto H, Toda M, Roeen Z et al. Protein S is protective in pulmonary fibrosis. J Thromb Haemost 2016; 14: 1588–1599.
Acknowledgements
This research was supported in part by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Esteban C Gabazza), and research grants from Aqua Therapeutic Corporation. The funders had no role in study design, data analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
MY, KT and KY are employees of Aqua Therapeutics Company and ECG received a Grant for Research from Aqua Therapeutics. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Tomaru, A., Kobayashi, T., Hinneh, J. et al. Oligonucleotide-targeting periostin ameliorates pulmonary fibrosis. Gene Ther 24, 706–716 (2017). https://doi.org/10.1038/gt.2017.80
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2017.80
This article is cited by
-
The role of periostin in cardiac fibrosis
Heart Failure Reviews (2023)
-
Coal dust nanoparticles induced pulmonary fibrosis by promoting inflammation and epithelial-mesenchymal transition via the NF-κB/NLRP3 pathway driven by IGF1/ROS-mediated AKT/GSK3β signals
Cell Death Discovery (2022)
-
Inhibition of lung microbiota-derived proapoptotic peptides ameliorates acute exacerbation of pulmonary fibrosis
Nature Communications (2022)
-
Periostin function in communication with extracellular matrices
Journal of Cell Communication and Signaling (2018)