Abstract
Several vaccination trials are evaluating the modified vaccinia virus Ankara (MVA) as a delivery vector in various clinical settings. In this paper, we present the reevaluation of a therapeutic vaccination trial in human immunodeficiency virus (HIV)-1-infected individuals treated with highly active antiretroviral therapy using MVA-expressing HIV-1 nef. Immunogenicity of MVA-nef was assessed using multicolor flow cytometry. Vaccine-induced polyfunctionality and proliferative capacity, which are associated with nonprogressive HIV-1 infection, were detectable by combining two immune assays. By means of short-term polychromatic intracellular cytokine staining, we observed a significant increase in polyfunctional Nef-specific CD4 T cells expressing interferon-γ, interleukin (IL)-2 and CD154 after vaccination, whereas changes in the quality of CD8 T-cell response could not be observed. Only the additional use of a long-term polychromatic Carboxyfluorescein succinimidyl ester (CFSE)-based proliferation assay revealed vaccine-induced Nef-specific CD8, as well as CD4 T cells with proliferative capacity. The correlation between vaccine-induced IL-2 production by CD4 T cells and the increase in proliferating Nef-specific CD8 T cells suggests a causal link between these two functions. These results highlight the importance of combining sophisticated immunomonitoring tools to unravel concealed effects of immunological interventions and support the use of the poxvirus-derived MVA vector to stimulate highly functional HIV-1-specific T-cell responses. However, the clinical benefit of these functional T cells remains to be determined.
Similar content being viewed by others
Introduction
To date, with 33.2 million people infected with human immunodeficiency virus (HIV)-1 (http://www.unaids.org) and no vaccine available, the only effective remedy against HIV-1 infection is highly active antiretroviral therapy (HAART). Despite substantial benefits, HAART is unable to clear HIV-1 infection and the virus still persists in resting CD4 T cells.1 As interruption of the antiviral treatment inevitably leads to disease progression, lifelong HAART administration is required. Unfortunately, HAART administration is associated with serious side effects, long-term toxicity and selection of multidrug-resistant viral strains. In addition, in developing countries, high cost and the required lifelong adherence are major limitations for the diffusion of HAART. In this scenario, therapeutic vaccination may represent an alternative strategy that aims at inducing immunological competence and impeding or delaying progression to acquired immunodeficiency syndrome (AIDS).2, 3
In a previous phase I study, we showed that a modified vaccinia virus Ankara vector expressing HIV-1LAI-nef (MVA-nef) was safe and immunogenic in 10 HIV-1-infected individuals undergoing HAART. The participants received MVA-nef vaccination twice (week 0 and 2), with a third vaccination 14 weeks after the second administration (week 16). MVA-nef was able to elicit or increase Nef-specific CD4 T-cell responses in 8 out of 10 participants. Preexisiting CD8 T-cell responses were observed in all participants and a minor increase in their magnitude associated with the administration of the vaccine was observed only in two subjects. A simple interferon (IFN)-γ based intracellular cytokine staining (ICS) performed with freshly isolated cell material was used to characterize specific T-cell responses in the first-line analysis of the clinical trial.4 However, as recent advances in polychromatic flow-cytometry technology revealed that the sole evaluation of IFN-γ provides limited information on the quality of antigen-specific T-cell responses,5, 6, 7, 8 in this study, we used more sophisticated assays to evaluate complex functional patterns of T-cell immune response. A correlation between the presence of antigen-specific CD4 T cells coproducing multiple cytokines and nonprogressive chronic HIV-1 infection has been shown in several cross-sectional studies.9, 10, 11, 12 Multifunctional CD4 or CD8 T cells are further proposed to represent a correlate of vaccine-mediated protection against various infectious diseases.6, 13, 14, 15 Although initial studies characterized the expression profile of IFN-γ and interleukin (IL)-2,9, 10, 11 more recent studies have expanded the investigation and analyzed more complex functional patterns.8, 12 Along with the functional profile, the proliferative potential of antigen-specific T cells is believed to be a key factor in maintaining or restoring effective antiviral immunity.16, 17
Applying polychromatic flow cytometry, we performed a reevaluation on the samples collected during the MVA-nef vaccination trial to further investigate the T-cell responses elicited by the vaccine. We evaluated Nef-specific CD8 and CD4 T-cell responses at selected time points by polychromatic ICS assay and polychromatic CFSE-based proliferation assay. As the original phase I study was not placebo controlled, we included a control group of 13 HAART-treated, HIV-1-infected individuals with similar clinical characteristics. As longitudinal control, we monitored the T-cell response toward the HIV-1 protein Tat throughout the course of the study. Tat was chosen because of similarity in features of the Nef and Tat protein with regard to their early expression. Our previous investigation suggested an innovative combination of functional markers.18 We studied the secretion of IFN-γ, representing the reference marker to detect and quantify specific antiviral T-cell immune responses;19 the cytokine IL-2, which is associated with helper function and essential for proliferation of antigen-specific T cells and memory development;20, 21, 22, 23 the chemokine macrophage inflammatory protein (MIP)-1β, which has been shown to dominate HIV-1-specific immune responses;8, 18 the expression of the differentiation marker CD45RA, enabling a discrimination between effector and memory T cells;24 and the CD4 T-cell-specific activation marker CD154 (CD40L), which allows the interaction with CD40-expressing antigen-presenting cells and therefore represents a link between innate and adaptive immunity.25, 26, 27 The polychromatic CFSE-based proliferation assay allowed for simultaneous evaluation of the proliferative capacity in combination with the expression pattern of functional markers IFN-γ, IL-2 and MIP-1β in CD4 and CD8 T cells. The combination of the standard ICS and the CFSE-based proliferation assay proved to be extremely useful in examinig the qualitative and quantitative aspects of MVA-nef-induced anti-Nef-specific immunity.
Results
MVA-nef vaccination increases the magnitude of the total Nef-specific CD4 T-cell response
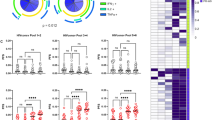
We used polychromatic flow cytometry to examine Nef-specific CD4 and CD8 T cells in cryopreserved peripheral blood mononuclear cell (PBMC) samples obtained from nine vaccine recipients. The analysis included PBMC collected before the first and after the second administration of the vaccine (Supplementary information: Table I). The ICS assay included the measurement of four functional markers (IFN-γ, IL-2, MIP-1β and CD154) and one differentiation marker (CD45RA) in CD4 and CD8 T-cell subsets. According to the differential expression of CD45RA, CD154, IFN-γ, IL-2 and MIP1β, 30 responding CD4 and CD8 T-cell sub-populations were identified. From each sub-population, the background as detected in an unstimulated control sample was subtracted. Only responses exceeding a predefined threshold level after background subtraction were considered. By summing up the frequency of CD4 or CD8 T cells of each unique T-cell population expressing at least one functional marker, we analyzed the magnitude of the total specific response (Figures 1a and c). The total frequency of Nef-specific CD4 T cells was significantly increased by two immunizations (P=0.0078; Wilcoxon signed-rank test) and was further boosted after the third administration in study subjects V01, V03, V04 and V08. The magnitude of CD4 T-cell response ranged from 0 to 0.204% (median 0.022%) before the administration of the vaccine, and from 0 to 1.028% (median 0.074%) and 0 to 0.967% (median 0.066%) after the second and the third immunizations, respectively. This corresponds to a 3.4-fold and a threefold increase in Nef-specific CD4 T-cells after the second and third immunizations, respectively. The total frequency of Nef-specific CD8 T cells did not show any significant variation temporally associated with the administration of the vaccine. The magnitude of the CD8 T-cell response ranged from 0.012 to 0.461% (median 0.27%) before the administration of the vaccine, and from 0.01 to 0.566 (median 0.206%) and 0.028 to 0.582% (median 0.249%) after the second and third immunizations, respectively. As a control, CD4 and CD8 T-cell responses to the regulatory HIV protein Tat were equally monitored, and significant variations temporally associated with the administration of the vaccine were not observed (data not shown). These data confirm our previous characterization of the T-cell response induced by the MVA-nef vaccine as measured by IFN-γ-based ICS.4 However, the weak CD8 T-cell response to the vaccine, previously observed in subjects V8 and V10, was not detected in this study. It is likely that the use of frozen material and the analysis of a limited number of time points might have affected the capacity to detect these weak changes in responses.
Total CD4 and CD8 T-cell responses throughout the course of the MVA-nef vaccination trial detected by polychromatic ICS and polychromatic CFSE-based proliferation assay. Total Nef-specific CD4 (a and b) and CD8 (c and d) T cells are shown before, and after two and three MVA-nef administrations as detected by ICS (a and c) and CFSE-based proliferation assays (b and d). Nine subjects were analyzed by ICS; the total response is calculated by summing up all responding subsets defined by IFN-γ, IL-2, MIP-1β secretion and CD154 expression. Eight subjects are analyzed by CFSE-based proliferation assay; the total response is calculated by summing up all responding subsets defined by IFN-γ, IL-2, MIP-1β secretion and a low CFSE staining level representing proliferative activity. All data are background subtracted. Minor responses are excluded using a predefined threshold system. To account for multiple comparison differences between the three time points, we applied nonparametric Friedman's test for three-way comparison, followed by a two-way Wilcoxon signed-rank test.
MVA-nef vaccination induces Nef-specific CD4 and CD8 T cells able to proliferate
As the proliferative potential of antigen-specific T cells is considered to be a key factor in maintaining or restoring effective antiviral immunity,16, 17, 28 it was of special interest to simultaneously evaluate the functional profile and the proliferative potential of Nef-specific CD4 and CD8 T cells during the course of the study. Thawed PBMCs were stained with CFSE, incubated with peptide pools for 5 days and, after restimulation, samples were stained according to our standard protocol, with the exception that the fluorescein isothiocyanate-conjugated (FITC) CD154 antibody was omitted so as not to interfere with the detection of CFSE. As there was not enough cell material available from study subject V10, the analysis of the proliferative potential included only eight study subjects. By summing up the frequency of CD4 or CD8 T cells of each unique T-cell population that was positive for at least one functional marker or was able to proliferate, we analyzed the magnitude of the total specific response (Figures 1b and d). The magnitude of the total Nef-specific CD4 T cells ranged from 0.015 to 0.467% (median 0.023) before the administration of the vaccine. After the second and third immunizations, the specific CD4 T-cell response ranged from 0.012 to 1.357% (median 0.04%) and from 0 to 0.825% (median 0.069%). Thus, the magnitude of the CD4 T-cell response detected by the proliferation assay was in the same quantitative range as the response detected by standard ICS (Figures 1a and b). Interestingly, the CFSE-based proliferation assay revealed a clear increase in functional and proliferating Nef-specific CD8 T cells after the second administration of MVA-nef (Figure 1d). The magnitude of total Nef-specific CD8 T cell responses ranged from 0 to 3.093% (median 0.152%) before the administration of the vaccine. After the second and third administrations, the CD8 T cells responding to Nef ranged from 0 to 8.698% (median 0.537%) and from 0 to 8.042% (median 0.355%), respectively. This corresponds to a 3.5-fold and a 2.3-fold increase in responding Nef-specific CD8 T-cells after the second and third immunizations, respectively. Thus, two doses of MVA-nef were able to enhance the proliferative capacity of specific CD8 T cells, but the third administration did not have a general boosting effect. In fact, in subjects V07 and V11, despite an important increase in proliferative response after two administrations of the vaccine, we observed a decrease in Nef-specific proliferating T cells after three vaccinations. T-cell responses specific to Tat were monitored throughout the study using the CFSE assay, showing no changes in association with the administration of MVA-nef (data not shown).
Figure 2 shows representative staining examples of ICS and proliferation assays performed before and after two vaccine administrations in study subject V11. CD4 T-cell responses to Nef were similarly detected using the two immune assays (Figures 2a and b), whereas the proliferation assay turned out to be dramatically more effective in detecting vaccine-induced CD8 T-cell responses in comparison with ICS (Figures 2c and d).
Representative CD4 and CD8 T-cell responses before and after two administrations of MVA-nef as measured by polychromatic ICS and polychromatic CFSE-based proliferation assay. Density plots show total CD4 (a and b) or CD8 (c and d) T cells in gray, overlaid by total responding, marker-expressing cells in violet. ICS assay data are depicted in a and c, whereas CFSE-based proliferation assay data are depicted in b and d. In each panel, the upper plots show the time point before the vaccination (W-4) and the lower plots show the time point after two MVA-nef administrations (W 4 for the ICS assay and W 8 for the proliferation assay). In each panel, the plots on the left show unstimulated controls and the plots on the right show stimulated samples. Stimulation with Nef N terminus and Nef C terminus peptide pools are shown for CD4 and CD8 T cells, respectively. The figure represents data from subject V11, in which specific CD4 and CD8 T cells recognized the C terminus and the N terminus of Nef, respectively. Numbers indicated on the graphs are the % of total responding cells relative to the parent population (CD4 or CD8) after background subtraction.
These results highlight a differential capacity of the ICS and proliferation assays to reveal specific immune responses. In particular, the proliferation assay allowed the detection of CD8 T-cell responses elicited by the vaccine, not observed using the ICS assay. Taken together, these results indicate a higher sensitivity of the proliferation assay for the detection of CD8 T cells, but not for CD4 T cells. It is possible that only a minor proportion of the specific CD4 T cells are able to proliferate, whereas nonproliferating cells die during the 5-day-incubation period.
MVA-nef vaccination increases the grade of functionality of CD4 T cells
The breakdown of total Nef-specific response into specific functional categories as detected by the polychromatic ICS assay is shown in Figure 3 for the study cohort, as well as for the nonvaccinated control group. We first analyzed CD4 T-cell responses (Figures 3a and b). CD4 T cells responding to Nef were mainly CD45RA negative. Only a single subject showed a clear CD45RA-positive Nef-specific subset before the administration of the vaccine, which declined after the second vaccination and disappeared after the third administration of the vaccine. This suggests a shift toward the CD45RA-negative memory population in this specific individual. As it is not possible to draw conclusions from a single subject, CD45RA was excluded from further CD4 T-cell analysis. The analysis of the degree of functionality showed that before the administration of the vaccine, mono-, bi-, tri- and tetra-functional Nef-specific CD4 T cells accounted for 52.5, 29.7, 16.6 and 1.3% of the total response, respectively. After two vaccine administrations, tri- and tetra-functional specific T cells increased to 21.2 and 6.6% of the total CD4 response, respectively. After the third administration of the vaccine, we observed a further increase to 30.6 and 7.6% of the total responding CD4 T cells. In the 13 HIV-infected individuals under HAART used as a reference cohort, mono-, bi-, tri- and tetra-functional Nef-specific CD4 T cells accounted for 71.79, 15.66, 11.04 and 1.51% of the total response, respectively.
Functional breakdown of total CD4 and CD8 T-cell responses as measured by polychromatic ICS. Pie charts (a and c) and aligned dot plots (b and d) are shown for CD4 (a and b) and CD8 (c and d) T-cell responses of all study participants and of a nonvaccinated HIV-1-infected, HAART-treated control group. (a and c) Pie charts show the functional subsets grouped according to their grade of functionality. Pies are created using the SPICE program taking into account the total response of the 9 study subjects or the 13 nonvaccinated control subjects. Pies represent mean fractions of tetra- (red), tri- (green), bi- (blue) and mono-functional (gray) responding T cells. Pies are shown for the nonvaccinated control group (black border) and for the study group time points before (brown border), and after two (turquoise border) and three (orange border) vaccine administrations. CD4 T-cell functions taken into account were CD154 expression and IFN-γ, IL-2 and MIP-1β secretion. CD8 T-cell functions taken into account were IFN-γ, IL-2 and MIP-1β secretion. (b and d) Aligned dot plots show all possible combinations of 1–4 markers in absolute % of the parent populations in the nonvaccinated control group (black rectangles) and for the study group before (brown) and after two (turquoise) or three (orange) vaccinations. Markers taken into account are specified in the respective graph. Black lines indicate median values of T-cell responses of the 13 nonvaccinated controls and the nine study participants. To account for multiple comparisons between the three time points, we applied a nonparametric Friedman's test for three-way comparison, followed by a two-way Wilcoxon signed-rank test. P-values of significant differences are indicated on the graph in black. The nonvaccinated control group was compared with the study group using a Mann–Whitney test. P-values for significant differences are indicated on the graph in gray.
The breakdown into specific functional categories showed a significant increase in Nef-specific CD4 T cells expressing CD154, IFN-γ and IL-2 after the second (P=0.031) and third (P=0.023) administrations of the vaccine. Before vaccination, this tri-functional subset was detectable in four of nine subjects (V7, V8, V10 and V11). In these four subjects, we observed a strong increase (median increase relative to prevaccination: 4.66-fold; range: 2.91-fold to 12.42-fold) after the second vaccination and a 1.81-fold increase (range: 0 to 10.16-fold) after the third vaccination. The tri-functional fraction producing CD154, IFN-γ and IL-2 became detectable in six out of nine subjects (V1, V4, V7, V8, V10 and V11) after two vaccinations and in seven out of nine subjects (V1, V3, V4, V5, V8, V10 and V11) after three vaccinations. It is to be noted that in the control group, the tri-functional fraction producing CD154, IFN-γ and IL-2 was also significantly lower when compared with the post-second and post-third vaccination values of the study group (P=0.043 and P=0.0287, respectively), whereas it was found in a similar range when compared with prevaccination values.
A significant increase in Nef-specific CD4 T cells expressing CD154 and IFN-γ (P=0.031) was additionally observed after the second administration of the vaccine. After the second vaccination, values of this bi-functional subset were also significantly higher than those of the control group. CD4 T cells expressing CD154, IFN-γ, IL-2 and MIP-1β were detected only in one study subject (V10) before the administration of the vaccine. After two administrations of the vaccine, these tetra-functional T cells became detectable in three additional study subjects (V01, V04 and V11) and were strongly boosted in study subject V10. The comparison between the control group and the study cohort indicates that T-cell responses in the study group were representative of a normal cohort of HAART-treated HIV-1-infected individuals and it suggests that, after vaccination, T-cell responses were more functional than responses usually found in HIV-1-infected individuals under HAART.
Remarkably, Nef-specific CD154+ CD4 T-cell responses were increased in five patients after the second administration (median increment: 0.13%; range: 0.06%–0.87%) and in six patients after the third administration of MVA-nef (median increment: 0.11%; range: 0.02%–0.92%), with respect to prevaccination levels. This indicates an improved capacity of these CD4 T cells to interact with antigen-presenting cells.
The degree of functionality of Nef-specific CD8 T cells did not change after vaccination, and the breakdown of the response into specific functional categories did not show any significant variation associated with the administration of the vaccine (Figure 3d). Expression of CD45RA on CD8 T cells showed a memory phenotype that differed among individuals but was not influenced by the administration of MVA-nef. The comparison with the nonvaccinated control group in Figures 3c and d shows a similar frequency and distribution of functional subsets within the CD8 T-cell response between the study and control cohort.
Functional characteristics of proliferating Nef-specific CD4 and CD8 T cells
Nef-specific CD4 (Figure 4a) or CD8 (Figure 4b) T cells detected by proliferation assay were dissected into functional categories according to their differential expression of IFN-γ, IL-2, MIP-1β and the capacity to proliferate. The total amount of CFSE low Nef-specific CD4 T cells increased after the second administration of the vaccine, from a median of 0% (range: 0–0.022%) to 0.04% (range: 0–0.232%). The functional breakdown showed that these cells mainly produced IFN-γ and MIP-1β, but the increase was limited to few study subjects and did not reach statistical significance. On the contrary, we observed a strong effect of the vaccination on the capacity of Nef-specific CD8 T cells to proliferate. The total amount of CFSE low Nef-specific CD8 T cells ranged from 0 to 3.093% (median 0.152%) before the administration of the vaccine, and from 0 to 8.395 (median 0.482%) and 0 to 8.042% (median 0.2%) after the second and third immunizations, respectively. In addition, significant differences were observed in specific CD8 T cells holding a proliferative potential and producing MIP-1β alone or MIP-1β and IFN-γ after the second (P=0.016; Wilcoxon signed-rank test) and third administrations (P=0.023; Wilcoxon signed-rank test), respectively.
Functional breakdown of total CD4 and CD8 T-cell responses as measured by polychromatic CFSE-based proliferation assay. Aligned dot plots are shown for CD4 (a) and CD8 (b) T-cell responses. All possible combinations of 1–4 markers in absolute % of the parent populations (CD4 or CD8 T cells) before (brown) and after two (turquoise) or three (orange) vaccine administrations are shown. To account for multiple comparisons between the three time points, we applied a Friedman's test, followed by a Wilcoxon signed-rank test.
Correlation between CD4 T-cell responses and CD8 T-cell proliferative activity
In HIV-infected subjects, a causal link between IL-2 production by CD4 T cells and T-cell proliferative capacity has been proposed.20, 29 Therefore, we determined whether there was a correlation between the increase in Nef-specific CD4 T-cell response detected by ICS assay and the increase in the proliferation activity observed in Nef-specific CD8 T cells. The analysis included the eight study subjects who have been analyzed in both assays and compared the increase in responses observed after the second and third administration of the vaccine. After the second administration of MVA-nef, we proved a significant correlation between the increase in Nef-specific CD4 T cells coexpressing CD154, IFN-γ and IL-2 as detected by ICS assay and the increase in total Nef-specific CD8 T cells as detected by proliferation assay (P=0.022; Spearman's correlation test (data not shown)). After the third vaccination, we found a significant correlation between the increase in Nef-specific CD4 T cells coexpressing CD154, IFN-γ and IL-2 as detected by ICS assay, and the increase in total Nef-specific CD8 T cells as well as the proliferating MIP-1β+ IFN-γ+ subset as detected by proliferation assay (P=0.028 and P=0.022, respectively; Spearman's correlation test). In addition, correlation analysis showed a significant association between the increase in total IL-2 production by CD4 T cells detected by ICS assay with the increase in total proliferating CD8 T cells after two and three administrations of MVA-nef (P=0.022 and P=0.007, respectively; Spearman's correlation test).
Vaccine-induced polyfunctional CD4 T cells produce high quantities of cytokines
Polyfunctional T cells produce more cytokines at a single-cell level than mono- or bi-functional T cells.14, 15, 30 A high level of cytokine expression might account for an effective control of viral replication by polyfunctional T cells.6 To assess the mean fluorescence intensity (MFI) of polyfunctional Nef-specific CD4 T cells elicited by the vaccine, we grouped the responding subsets according to their grade of functionality and then calculated the relative MFI for the expression of IFN-γ, IL-2, CD154 and MIP-1β (Figure 5). The relative MFI values were calculated by dividing the MFI of the functional subsets by the MFI of nonfunctional CD4 T cells. Tetra-functional Nef-specific CD4 T cells expressed significantly higher levels of IFN-γ than did tri-, bi- and mono-functional cells. A similar pattern was observed for the expression of CD154, with the exception that tetra- and tri-functional T cells had similar relative MFI. The distribution of MFI for IL-2 expression was identical to that observed for CD154, but as a small number of bi- and mono-functional CD4 T cells expressed IL-2, differences were not statistically significant. Finally, MIP-1β expression was significantly lower in mono-functional than in tetra-, tri- and bi-functional responding CD4 T cells, but among tetra-, tri- and bi-functional CD4 T cells, no differences were observed. These data suggest that the Nef-specific polyfunctional CD4 T cells observed in our study present the typical phenotype of highly functional effector cells. The overlaid dot plots in Figure 5b show the florescence intensity of mono-, bi-, tri- and tetra-functional cells in the representative study subject V10 during the course of the study. After the administration of the vaccine, we observed a clear increase in Nef-specific CD4 T cells with a polyfunctional phenotype clearly expressing highest amounts of IFN-γ, IL2 and CD154 on a per-cell basis.
Characterization of polyfunctional CD4 T cells. (a) CD4 T cells responding to stimulation with Nef-derived peptide pools are grouped according to their grade of functionality and expression of IFN-γ, IL-2, MIP-1β or CD154 into tetra- (red), tri- (green), bi- (blue) and mono-functional (gray). Relative MFI values were calculated by dividing the MFI of the functional subsets by the MFI of nonfunctional CD4 T cells. Each point represents a positive CD4 T-cell response detected with Nef peptide pools covering either the N terminus or the C terminus of the Nef protein. To account for multiple comparisons, differences between the functional groups were assessed by nonparametric Kruskal–Wallis test for four-way comparison, followed by a pairwise Mann–Whitney test. (b) Representative flow cytometric data before vaccination (W-6), after the second vaccination (W 4) and after the third vaccination (W20) from subject V10 are depicted in overlaid dot plots. Mono-, bi-, tri- and tetra-functional Nef-specific CD4 T cells are indicated using the same color coding as for the graphs in (a). Asterisks indicate the level of significance, with ***P<0.0001; **P<0.001; *P<0.05.
Discussion
Therapeutic vaccination may represent a valuable tool for achieving viral control in HIV-1-infected individuals by boosting the virus-specific immune response. Various HIV-1 immunogens have been tested in HIV-1-infected individuals and were able to stimulate specific CD4 and CD8 T-cell responses.4, 31, 32, 33, 34, 35, 36, 37 Vaccine-specific immune responses were evaluated with immune assays such as enzyme-linked immunosorbent spot (ELISPOT),35, 37, 38 proliferation assay31, 32, 35, 38 and ICS.33, 34, 35, 38 However, at present, between these assays, polychromatic ICS is regarded as a very informative immunoassay, as it provides simultaneous information on multiple markers measured at the single-cell level. Indeed, polychromatic ICS is becoming increasingly more popular in assessing HIV-specific immune responses in different clinical settings.
In this study, by means of polychromatic flow cytometry, we reexamined HIV-1-specific T-cell responses throughout the course of a therapeutic vaccination trial that used MVA-nef as immunogen.4 The insight gathered in this study exceeded the information obtained in our first evaluation, in which a simple IFN-γ-based immune assay was applied to freshly isolated PBMCs. Indeed, we observed that CD4 T cells elicited by MVA-nef were polyfunctional and were able to proliferate. In addition, using a long-term polychromatic proliferation assay, we revealed specific CD8 T-cell responses induced by the vaccine.
MVA-nef was able to significantly increase the frequency of Nef-specific CD4 T cells expressing CD154 and IFN-γ, as well as CD4 T cells expressing CD154, IFN-γ and IL-2. In addition, Nef-specific CD4 T cells expressing CD154, IFN-γ, IL-2 and MIP-1β were induced in several subjects. As shown by MFI analysis, vaccine-elicited polyfunctional CD4 T cells showed increased production of IFN-γ, CD154 and IL-2 on a per-cell level, whereas the quantity of produced MIP-1β was independent from the grade of functionality. These data indicate that the polyfunctional CD4 T cells observed in this study are possibly similar to polyfunctional T cells that have been associated with successful immune responses in several clinical settings.6, 14, 15 Nef-specific CD4 T cells have been shown to produce higher amounts of IFN-γ in nonprogressive than in progressive infection, indicating a higher grade of functionality.39 Furthermore, polyfunctional responses toward different HIV antigens including Nef have been found in rhesus monkeys protected from mucosal simian immunodeficiency virus challenge by vaccination.40 In addition to improvements in effector functions, MVA-nef was able to induce specific CD4 and especially CD8 T cells endowed with proliferative potential. Although functional CD4 T-cell responses and the proliferative potential of T cells were significantly increased after the second vaccine administration, there was no clear additional boosting effect after the third administration of MVA-nef. Preexisting immunity to vaccinia virus (eight out of nine subjects were vaccinated against smallpox) and MVA-induced vector immunity might be an explanation for this observation. In fact, in a previous study,41 we observed strong MVA-specific cellular and humoral immune responses after vaccination with MVA-nef. Overall, the MVA-nef vaccine was able to modify the Nef-specific response toward a T-cell phenotype associated with a good prognosis of HIV-1 disease progression. IL-2 secretion by CD4 T cells is associated with control of HIV-1 replication in infected individuals and is related to the proliferative potential of CD4 and CD8 T cells.20, 29, 42 HIV-1 viremia, in particular, impedes the establishment of IL-2-producing HIV-1-specific memory CD4 T cells endowed with proliferative capacity.43 Several lines of evidence indicate that the proliferative capacity is maintained in primary and nonprogressive HIV-1 infection and impaired in chronic progressive infection.16, 20, 44 Whether the proliferative impairment is reversible by endogenous or exogenous IL-217, 20, 29 is still a matter of discussion. Whatever the case, the MVA-nef vaccine was able to increase the number of Nef-specific IL-2-producing CD4 T cells and Nef-specific CD4 and CD8 T cells capable of proliferating ex vivo. This indicates an improved capacity of effector CD4 and particularly CD8 T cells to expand in response to HIV-1 antigens. In addition, after vaccination, a significant correlation between the increase in IL-2 production by specific CD4 T cells and the increase in CD8 T-cell proliferative activity has been observed. The statistical correlation suggests the possibility of a causal link between the two functions. A valid proof for this hypothesis would require further experiments, for which cell material from the study subjects is not available anymore.
The induction of CD154+ CD4 T cells is typically impaired in HIV-1 infection,45 and in progressive infection, CD154-expressing CD4 T cells are selectively depleted.26 Thus, the vaccine-induced increase in Nef-specific CD4 T cells expressing CD154 suggests a reversion of the impaired CD154 expression and likely an improved interaction potential between CD4 T cells and antigen-presenting cells.
Despite improved knowledge of the immune system and significant advancement in immune-monitoring technologies, it remains unclear which elements of the immune system need to be stimulated to provide protection against HIV-1 infection and improve viral control in already HIV-1-infected individuals. Polyfunctional CD8 and CD4 T cells, which are usually associated with successful immune responses, have been detected in healthy subjects vaccinated with a defective adenovirus vaccine vector.46 However, the same vaccine was ineffective in protecting against infection in a proof-of-concept phase IIb trial.47 Therefore, although the MVA-nef vaccine was able to elicit responses normally associated with slow HIV-1 disease progression in infected individuals and with naturally controlled infectious diseases, efficacy studies are warranted to assess eventual clinical benefits provided by the vaccine. In this regard, future immunization studies should incorporate a period of HAART discontinuation to assess whether vaccine-elicited responses are associated with improved control of viral replication.
Recently, it has been shown that, compared with New York vaccinia virus, MVA is able to stimulate both CD4 and CD8 T-cell responses in an equilibrated way in rhesus macaques primed with a DNA vaccine.48 In our study, we detected more CD4 than CD8 T cells specific to Nef. As the expansion of CD4 T cells requires sustained antigen presentation in contrast to CD8 T cells,49 it can be suggested that CD4 T-cell expansion is favored in chronic infection in which there is a continuous presence of the antigen. In addition, in induction of apoptosis in cells infected by the MVA vector, followed by MHC class II restricted antigen cross-presentation might be a possible reason for the preferential elicitation of specific CD4 T cells. Nevertheless, the mechanism for the differential stimulation of CD4 and CD8 T-cell responses remains to be clarified by mechanistic studies.
From a methodological point of view, the present study highlighted how the use of the polychromatic CFSE-based proliferation assay augmented the perceived immunogenicity of the MVA-nef vaccine. In fact, both the ex vivo IFN-γ-based ICS performed in a previous study4 and the polychromatic ICS performed in this study underestimated the vaccine-induced Nef-specific CD8 T-cell responses clearly detected by the proliferation assay. Recently, Winstone et al.50 used a cultured IFN-γ ELISPOT assay to reexamine vaccine-induced T-cell responses in trial IAVI-006. The cultured IFN-γ ELISPOT assay detected five times more vaccine-induced responses in study participants as compared with the classical ELISPOT.51, 52 The proliferation assay used in our study and the cultured IFN-γ ELISPOT assay used to evaluate the IAVI-006 trial have, in common, several days of incubation in the presence of antigen, suggesting that an expansion step may be required to reveal specific responses otherwise undetectable. The polychromatic CFSE-based proliferation assay simultaneously assessing functional markers and proliferative capacity represents, therefore, an innovative and sensitive immunoassay, well suited to reveal concealed effects of immunological interventions that are undetectable using simple IFN-γ-based ICS as in the original study4 or complex polychromatic ICS without prolonged stimulation periods.
Safety and immunogenicity of vaccines based on recombinant poxviruses have been documented extensively. MVA is a highly attenuated strain of vaccinia virus widely used as a safe viral vector to deliver antigens;53 it does not replicate in most mammalian cells54 and is thus considered as a good candidate vaccine for individuals with a compromised immune system, such as HIV-1-infected patients. Our data, along with previous reports in which an HIV-1 gag/multiepitope immunogen was delivered by the MVA vector,38 show the capacity of MVA to improve the quality of the HIV-1-specific immune response in infected individuals. Therefore, a general capacity of the MVA vector to induce highly functional responses against heterologous genetic inserts can be suggested. In our study, we could show the elicitation of T-cell responses against the nonstructural, highly conserved and early-expressed Nef protein encoded by the vector.
In conclusion, the MVA-nef vaccine was able to change the quality and quantity of the Nef-specific immune response in HIV-1-infected subjects under HAART. These results support the use of the poxvirus-derived MVA vector to stimulate and improve anti-HIV-1 T-cell responses. Whether highly functional HIV-1-specific CD4 and CD8 T cells endowed with proliferative capacity will be clinically relevant in HIV-1 infection remains to be determined. Further, clinical trials designed to test immunogenicity in combination with the therapeutic efficacy of MVA-nef vaccination will help to define the clinical relevance of the observed immune responses.
Materials and methods
Study samples
In all, 10 HIV-1-infected individuals under HAART were vaccinated with MVA-nef to assess safety and immunogenicity.4 According to available cryopreserved samples, PBMCs obtained from nine individuals were reexamined in this study. Prevaccination samples were not available from study subject V02 and this participant was excluded from the present evaluation. Subject V10 was analyzed only by polychromatic ICS, as not enough material was available to perform the proliferation assay. Eight out of nine participants had undetectable viral load. In subject V3, the viral load was 8710 RNA copies per ml. CD4 T-cell counts ranged from 407 to 1421 cells per mm3 with a median of 549 cells per mm.3 All the nine subjects received three subcutaneous immunizations of 5 × 108 plaque-forming units of MVA-nef at week 0, 2 and 16.
A control group of 13 HIV-1-infected, HAART-treated individuals was included in this study. Their clinical characteristics were similar to those of vaccinated individuals. All individuals in the control group had undetectable viral load. The CD4 T-cell counts ranged from 334 to 1100 cells per mm3, with a median of 603 cells per mm3.
Peptides
Three different HIV-1-derived peptide pools were used to stimulate PBMCs: (1) variable length overlapping peptides spanning the 1–101 region of HIV-1 Bru Nef (Nef N terminus peptide pool); (2) variable length overlapping peptides spanning the 96–205 region of HIV-1 Bru Nef (Nef C terminus peptide pool); and (3) variable length overlapping peptides spanning HIV-1 BH10 Tat. The peptide sets were designed as previously described55 and validated for use in cytokine secretion T-cell ELISPOT, as well as in intracellular cytokine staining assay within the European Commission-sponsored AIDS Vaccine Integrated project consortium. Peptides were obtained from the Centre for AIDS Reagents, National Institute for Biological Standards and Control, Hertfordshire, UK.
Intracellular cytokine staining
After thawing, 106 cryopreserved PBMCs were resuspended in 150 μl culture medium RPMI 1640 (Cambrex, Taufkirchen, Germany), supplemented with 10% fetal calf serum (Biochrom AG, Berlin, Germany) and 1% PenStrep (Cambrex). Peptide-stimulated and mock-stimulated samples were run in parallel to define the background. Stimulation was performed with 0.4 μg peptide per 106 cells in the presence of 1.3 μg ml−1 anti-CD28 and 1.3 μg ml−1 anti-CD49d costimulatory antibodies (Becton Dickinson, Heidelberg, Germany). A mock-stimulated sample (containing only costimulatory antibodies) was run for each patient and time point. After 60 min of incubation, 10 μg ml−1 of Brefeldin A (Sigma-Aldrich, Taufkirchen, Germany) was added to the cell suspension and the incubation was carried out for an additional 4 h. Stimulated cells were labeled with the photoreactive fluorescent viability marker ethidium monoazide (Molecular Probes/Invitrogen, Darmstadt, Germany), fixed and permeabilized using the BD Cytofix/CytopermTM Kit (BD Biosciences, Heidelberg, Germany). Cells were stained with the following fluorochrome-conjugated antibodies: CD3-AmCyan, CD8-PacB, CD4-PerCP, CD45RA-PECy7, CD154-FITC, IFN-γ-Al700, IL-2-Allophycocyanin (APC) and MIP1β-PE. All antibodies were from BD Biosciences, except CD8-PacB (DAKO Cytomation, Hamburg, Germany). Samples were acquired using an LSRII flow cytometer (BD Biosciences) equipped with a high-throughput system. Analysis was performed using FlowJo version 8.5.3 (Tree Star, Ashland, OR, USA). Gating strategy was described previously18 and is shown for the functional markers in the Supplementary information (Figure 1—gating strategy). According to the differential expression of CD45RA, CD154, IFN-γ, IL-2 and MIP1β, 30 responding CD4 and CD8 T-cell sub-populations were identified. An individual threshold level for each sub-population was calculated after background subtraction using SPICE software (Mario Roederer, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health). This is important for correct data evaluation, as the nonspecific background becomes very low (sometimes equal to zero) when examining combinations of three or more positive functions. In contrast, background is higher for single positive responses, particularly for the chemokine MIP-1β. The threshold level was defined for each functional combination as the 90th percentile of the distribution of negative values from 147 stimulated samples derived from 37 HIV-1-infected individuals, including the study participants. Values lower than the respective individual threshold level were set to 0. Furthermore, a general threshold of 0.005% was applied for all CD8+ and CD4+ T-cell subsets to exclude minor responses. As a result, threshold ranged from 0.005% to 0.11%, according to the different sub-populations.
CFSE proliferation assay
After thawing, PBMCs were resuspended in 0.1 μM CFSE (Molecular Probes/Invitrogen). Cells were incubated for 10 min at 37 °C in the dark, rinsed in complete medium and plated at 200 μl per well (106 cells per 200 μl). Peptide antigens and costimulatory antibodies were added as for the ICS, and cells were cultured for 5 days in complete medium. At day 5, cells were resuspended in 200 μl culture media and restimulated applying the same procedure and conditions as for the ICS. After ethidium monoazide staining and fixation/permeabilization, cells were stained with the fluorochrome-conjugated antibodies used in the ICS protocol. The CD154-FITC antibody was omitted so as not to interfere with the detection of CFSE. Acquisition and sample analysis were performed in concordance with the ICS protocol. Functional markers and ex vivo proliferative activity, defined by low CFSE staining level, were gated according to the gating strategy depicted in the Supplementary information (Figure 1—gating strategy). According to the differential expression of CD45RA, IFN-γ, IL-2, MIP1β and the CFSE staining level, 30 responding CD4 and CD8 T-cell sub-populations were identified. An individual threshold level for each sub-population was calculated following background subtraction using SPICE software as described in the ICS section. The threshold level was defined for each functional combination as the 95th percentile of the distribution of negative values from 201 samples from 16 HIV-1-infected individuals, including the study participants. A higher percentile was chosen for the proliferation assay in comparison with ICS, as in long-term assays, T-cell responses, as well as background levels, tend to have a higher variability than in short-term assays. A general threshold of 0.005% for all CD8+ and CD4+ T-cell subsets was applied to exclude minor responses.
Data processing and statistical analysis
SPICE version 4.1.5 and Prism version 5.01 (GraphPad Software Inc., San Diego, CA, USA) were used for graphical representation and statistical analysis.
Nonparametric statistical tests were applied in all cases. To account for multiple comparisons, nonparametric Friedman's tests, followed by pairwise post hoc Wilcoxon signed-rank tests, were used to assess significance of change in values between study time points. Spearman's correlation test was used for correlation analysis. A confidence interval of 95% was used for all statistical considerations.
References
Noe A, Plum J, Verhofstede C . The latent HIV-1 reservoir in patients undergoing HAART: an archive of pre-HAART drug resistance. J Antimicrob Chemother 2005; 55: 410–412.
Gudmundsdotter L, Sjodin A, Bostrom AC, Hejdeman B, Theve-Palm R, Alaeus A et al. Therapeutic immunization for HIV. Springer Semin Immunopathol 2006; 28: 221–230.
Dorrell L . Therapeutic immunization strategies for the control of HIV-1. Expert Rev Vaccines 2005; 4: 513–520.
Cosma A, Nagaraj R, Buhler S, Hinkula J, Busch DH, Sutter G et al. Therapeutic vaccination with MVA-HIV-1 nef elicits Nef-specific T-helper cell responses in chronically HIV-1 infected individuals. Vaccine 2003; 22: 21–29.
Makedonas G, Betts MR . Polyfunctional analysis of human T cell responses: importance in vaccine immunogenicity and natural infection. Springer Semin Immunopathol 2006; 28: 209–219.
Seder RA, Darrah PA, Roederer M . T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol 2008; 8: 247–258.
Elias D, Akuffo H, Britton S . PPD induced in vitro interferon gamma production is not a reliable correlate of protection against Mycobacterium tuberculosis. Trans R Soc Trop Med Hyg 2005; 99: 363–368.
Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 2006; 107: 4781–4789.
Emu B, Sinclair E, Favre D, Moretto WJ, Hsue P, Hoh R et al. Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J Virol 2005; 79: 14169–14178.
Harari A, Petitpierre S, Vallelian F, Pantaleo G . Skewed representation of functionally distinct populations of virus-specific CD4T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood 2004; 103: 966–972.
Boaz MJ, Waters A, Murad S, Easterbrook PJ, Vyakarnam A . Presence of HIV-1 Gag-specific IFN-gamma+IL-2+ and CD28+IL-2+ CD4T cell responses is associated with nonprogression in HIV-1 infection. J Immunol 2002; 169: 6376–6385.
Kannanganat S, Kapogiannis BG, Ibegbu C, Chennareddi L, Goepfert P, Robinson HL et al. Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4T cells coexpressing three cytokines. J Virol 2007; 81: 12071–12076.
Beveridge NE, Price DA, Casazza JP, Pathan AA, Sander CR, Asher TE et al. Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur J Immunol 2007; 37: 3089–3100.
Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 2007; 13: 843–850.
Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med 2007; 204: 1405–1416.
Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol 2002; 3: 1061–1068.
Jagannathan P, Osborne CM, Royce C, Manion MM, Tilton JC, Li L et al. Comparisons of CD8+ T cells specific for human immunodeficiency virus, hepatitis C virus, and cytomegalovirus reveal differences in frequency, immunodominance, phenotype, and interleukin-2 responsiveness. J Virol 2009; 83: 2728–2742.
Kutscher S, Dembek CJ, Allgayer S, Heltai S, Stadlbauer B, Biswas P et al. The intracellular detection of MIP-1beta enhances the capacity to detect IFN-gamma mediated HIV-1-specific CD8 T-cell responses in a flow cytometric setting providing a sensitive alternative to the ELISPOT. AIDS Res Ther 2008; 5: 22.
Pantaleo G, Harari A . Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat Rev Immunol 2006; 6: 417–423.
Lichterfeld M, Kaufmann DE, Yu XG, Mui SK, Addo MM, Johnston MN et al. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J Exp Med 2004; 200: 701–712.
Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev 2006; 211: 81–92.
MacLeod MK, McKee A, Crawford F, White J, Kappler J, Marrack P . CD4 memory T cells divide poorly in response to antigen because of their cytokine profile. Proc Natl Acad Sci USA 2008; 105: 14521–14526.
Nomura LE, Emu B, Hoh R, Haaland P, Deeks SG, Martin JN et al. IL-2 production correlates with effector cell differentiation in HIV-specific CD8+ T cells. AIDS Res Ther 2006; 3: 18.
Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A . Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999; 401: 708–712.
van Kooten C, Banchereau J . CD40-CD40 ligand. J Leukoc Biol 2000; 67: 2–17.
Kornbluth RS . The emerging role of CD40 ligand in HIV infection. J Leukoc Biol 2000; 68: 373–382.
Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M et al. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med 2005; 11: 1118–1124.
Das A, Hoare M, Davies N, Lopes AR, Dunn C, Kennedy PT et al. Functional skewing of the global CD8T cell population in chronic hepatitis B virus infection. J Exp Med 2008; 205: 2111–2124.
Iyasere C, Tilton JC, Johnson AJ, Younes S, Yassine-Diab B, Sekaly RP et al. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J Virol 2003; 77: 10900–10909.
Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR . Multiple-cytokine-producing antiviral CD4T cells are functionally superior to single-cytokine-producing cells. J Virol 2007; 81: 8468–8476.
Robbins GK, Addo MM, Troung H, Rathod A, Habeeb K, Davis B et al. Augmentation of HIV-1-specific T helper cell responses in chronic HIV-1 infection by therapeutic immunization. Aids 2003; 17: 1121–1126.
Levy Y, Gahery-Segard H, Durier C, Lascaux AS, Goujard C, Meiffredy V et al. Immunological and virological efficacy of a therapeutic immunization combined with interleukin-2 in chronically HIV-1 infected patients. Aids 2005; 19: 279–286.
Dorrell L, Yang H, Ondondo B, Dong T, di Gleria K, Suttill A et al. Expansion and diversification of virus-specific T cells following immunization of human immunodeficiency virus type 1 (HIV-1)-infected individuals with a recombinant modified vaccinia virus Ankara/HIV-1 Gag vaccine. J Virol 2006; 80: 4705–4716.
Moss RB, Webb E, Giermakowska WK, Jensen FC, Savary JR, Wallace MR et al. HIV-1-Specific CD4 helper function in persons with chronic HIV-1 infection on antiviral drug therapy as measured by ELISPOT after treatment with an inactivated, gp120-depleted HIV-1 in incomplete Freund's adjuvant. J Acquir Immune Defic Syndr 2000; 24: 264–269.
Valor L, Navarro J, Carbone J, Rodriguez-Sainz C, Gil J, Lopez F et al. Immunization with an HIV-1 immunogen induces CD4+ and CD8+ HIV-1-specific polyfunctional responses in patients with chronic HIV-1 infection receiving antiretroviral therapy. Vaccine 2008; 26: 2738–2745.
Greenough TC, Cunningham CK, Muresan P, McManus M, Persaud D, Fenton T et al. Safety and immunogenicity of recombinant poxvirus HIV-1 vaccines in young adults on highly active antiretroviral therapy. Vaccine 2008; 26: 6883–6893.
Harrer E, Bauerle M, Ferstl B, Chaplin P, Petzold B, Mateo L et al. Therapeutic vaccination of HIV-1-infected patients on HAART with a recombinant HIV-1 nef-expressing MVA: safety, immunogenicity and influence on viral load during treatment interruption. Antivir Ther 2005; 10: 285–300.
Ondondo BO, Yang H, Dong T, di Gleria K, Suttill A, Conlon C et al. Immunisation with recombinant modified vaccinia virus Ankara expressing HIV-1 gag in HIV-1-infected subjects stimulates broad functional CD4+ T cell responses. Eur J Immunol 2006; 36: 2585–2594.
Pancre V, Delhem N, Yazdanpanah Y, Delanoye A, Delacre M, Depil S et al. Presence of HIV-1 Nef specific CD4T cell response is associated with non-progression in HIV-1 infection. Vaccine 2007; 25: 5927–5937.
Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med 2009; 15: 293–299.
Cosma A, Nagaraj R, Staib C, Diemer C, Wopfner F, Schatzl H et al. Evaluation of modified vaccinia virus Ankara as an alternative vaccine against smallpox in chronically HIV type 1-infected individuals undergoing HAART. Aids Res Hum Retroviruses 2007; 23: 782–793.
Harari A, Dutoit V, Cellerai C, Bart PA, Du Pasquier RA, Pantaleo G . Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol Rev 2006; 211: 236–254.
Younes SA, Yassine-Diab B, Dumont AR, Boulassel MR, Grossman Z, Routy JP et al. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J Exp Med 2003; 198: 1909–1922.
Day CL, Kiepiela P, Leslie AJ, van der Stok M, Nair K, Ismail N et al. Proliferative capacity of epitope-specific CD8 T-cell responses is inversely related to viral load in chronic human immunodeficiency virus type 1 infection. J Virol 2007; 81: 434–438.
Subauste CS, Subauste A, Wessendarp M . Role of CD40-dependent down-regulation of CD154 in impaired induction of CD154 in CD4(+) T cells from HIV-1-infected patients. J Immunol 2007; 178: 1645–1653.
Cox KS, Clair JH, Prokop MT, Sykes KJ, Dubey SA, Shiver JW et al. DNA gag/adenovirus type 5 (Ad5) gag and Ad5 gag/Ad5 gag vaccines induce distinct T-cell response profiles. J Virol 2008; 82: 8161–8171.
Steinbrook R . One step forward, two steps back—will there ever be an AIDS vaccine? N Engl J Med 2007; 357: 2653–2655.
Mooij P, Balla-Jhagjhoorsingh SS, Koopman G, Beenhakker N, van Haaften P, Baak I et al. Differential CD4+ versus CD8+ T-cell responses elicited by different poxvirus-based human immunodeficiency virus type 1 vaccine candidates provide comparable efficacies in primates. J Virol 2008; 82: 2975–2988.
Obst R, van Santen HM, Mathis D, Benoist C . Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med 2005; 201: 1555–1565.
Winstone N, Guimaraes-Walker A, Roberts J, Brown D, Loach V, Goonetilleke N et al. Increased detection of proliferating, polyfunctional, HIV-1-specific T cells in DNA-modified vaccinia virus Ankara-vaccinated human volunteers by cultured IFN-gamma ELISPOT assay. Eur J Immunol 2009; 39: 975–985.
Guimaraes-Walker A, Mackie N, McCormack S, Hanke T, Schmidt C, Gilmour J et al. Lessons from IAVI-006, a phase I clinical trial to evaluate the safety and immunogenicity of the pTHr.HIVA DNA and MVA.HIVA vaccines in a prime-boost strategy to induce HIV-1 specific T-cell responses in healthy volunteers. Vaccine 2008; 26: 6671–6677.
Hanke T, Goonetilleke N, McMichael AJ, Dorrell L . Clinical experience with plasmid DNA- and modified vaccinia virus Ankara-vectored human immunodeficiency virus type 1 clade A vaccine focusing on T-cell induction. J Gen Virol 2007; 88 (Part 1): 1–12.
Slifka MK . The future of smallpox vaccination: is MVA the key? Med Immunol 2005; 4: 2.
Drexler I, Heller K, Wahren B, Erfle V, Sutter G . Highly attenuated modified vaccinia virus Ankara replicates in baby hamster kidney cells, a potential host for virus propagation, but not in various human transformed and primary cells. J Gen Virol 1998; 79 ( Part 2): 347–352.
Vardas E, Butto S, Glashoff R, Malnati MS, Poli G, Clerici M . Preparing for phase II/III HIV vaccine trials in Africa. Microbes Infect 2005; 7: 1436–1444.
Acknowledgements
This work was supported by the AIDS Vaccine Integrated Project (contract LSHP-CT-2004-503487). Peptides were provided by the Centre for AIDS reagents through the EU Program EVA Centre for AIDS Reagents, NIBSC, UK. We thank Dolores Schendel for her valuable support and Georg Gasteiger for critical reading of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Kutscher, S., Allgayer, S., Dembek, C. et al. MVA-nef induces HIV-1-specific polyfunctional and proliferative T-cell responses revealed by the combination of short- and long-term immune assays. Gene Ther 17, 1372–1383 (2010). https://doi.org/10.1038/gt.2010.90
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2010.90