Abstract
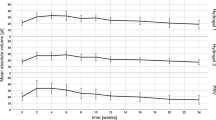
Nucleic acid-based therapies hold great promise for treatment of skin disorders if delivery challenges can be overcome. To investigate one mechanism of nucleic acid delivery to keratinocytes, a fixed mass of expression plasmid was intradermally injected into mouse footpads in different volumes, and reporter expression was monitored by intravital imaging or skin sectioning. Reporter gene expression increased with higher delivery volumes, suggesting that pressure drives nucleic acid uptake into cells after intradermal injections similar to previously published studies for muscle and liver. For spatiotemporal analysis of reporter gene expression, a dual-axis confocal (DAC) fluorescence microscope was used for intravital imaging following intradermal injections. Individual keratinocytes expressing hMGFP were readily visualized in vivo and initially appeared to preferentially express in the stratum granulosum and subsequently migrate to the stratum corneum over time. Fluorescence microscopy of frozen skin sections confirmed the patterns observed by intravital imaging. Intravital imaging with the DAC microscope is a noninvasive method for probing spatiotemporal control of gene expression and should facilitate development and testing of new nucleic acid delivery technologies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Abbreviations
- DAC:
-
dual-axis confocal
- CBL:
-
click beetle luciferase
- hMGFP:
-
humanized monster green fluorescent protein
- siRNA:
-
small-interfering RNA
- RNAi:
-
RNA interference
- Ubc:
-
ubiquitin C
- eIF4A1:
-
eukaryotic translation initiation factor 4A, isoform 1
- EF1a:
-
elongation factor 1a
- BLI:
-
bioluminescence imaging
- i.d.:
-
intradermal
References
Khavari PA, Rollman O, Vahlquist A . Cutaneous gene transfer for skin and systemic diseases. J Int Med 2002; 252: 1–10.
Cassaday RD, Sondel PM, King DM, Macklin MD, Gan J, Warner TF et al. A phase I study of immunization using particle-mediated epidermal delivery of genes for gp100 and GM-CSF into uninvolved skin of melanoma patients. Clin Cancer Res 2007; 13: 540–549.
Pilla L, Valenti R, Marrari A, Patuzzo R, Santinami M, Parmiani G et al. Vaccination: role in metastatic melanoma. Expert Rev Anticancer Ther 2006; 6: 1305–1318.
Lewin AS, Glazer PM, Milstone LM . Gene therapy for autosomal dominant disorders of keratin. J Investig Dermatol Symp Proc 2005; 10: 47–61.
Tamai K, Kaneda Y, Uitto J . Molecular therapies for heritable blistering diseases. Trends Mol Med 2009; 15: 285–292.
Leachman SA, Hickerson RP, Hull PR, Smith FJ, Milstone LM, Lane EB et al. Therapeutic siRNAs for dominant genetic skin disorders including pachyonychia congenita. J Dermatol Sci 2008; 51: 151–157.
Leachman SA, Hickerson RP, Schwartz ME, Bullough EE, Hutcherson SL, Boucher KM et al. First-in-human mutation-targeted siRNA Phase Ib trial of an inherited skin disorder. Mol Ther 2009; 18: 442–446.
Hengge UR, Walker PS, Vogel JC . Expression of naked DNA in human, pig, and mouse skin. J Clin Invest 1996; 97: 2911–2916.
Sawamura D, Akiyama M, Shimizu H . Direct injection of naked DNA and cytokine transgene expression: implications for keratinocyte gene therapy. Clin Exp Dermatol 2002; 27: 480–484.
Ghazizadeh S, Doumeng C, Taichman LB . Durable and stratum-specific gene expression in epidermis. Gene Therapy 2002; 9: 1278–1285.
Ghazizadeh S, Katz AB, Harrington R, Taichman LB . Lentivirus-mediated gene transfer to human epidermis. J Investig Dermatol Symp Proc 2004; 9: 269–275.
Yang CH, Shen SC, Lee JC, Wu PC, Hsueh SF, Lu CY et al. Seeing the gene therapy: application of gene gun technique to transfect and decolour pigmented rat skin with human agouti signalling protein cDNA. Gene Therapy 2004; 11: 1033–1039.
Yang NS, Burkholder J, McCabe D, Neumann V, Fuller D . Particle-mediated gene delivery in vivo and in vitro. Curr Protoc Hum Genet 2001 Chapter 12: Unit 12.6, pp 1–14.
Holzbach T, Vlaskou D, Neshkova I, Konerding MA, Wortler K, Mykhaylyk O et al. Non-viral VEGF gene therapy—magnetofection of acoustically active magnetic lipospheres (‘Magnetobubbles’) increases tissue-survival in an oversized skin flap model. J Cell Mol Med 2010; 14: 587–599.
Wolff JA, Budker V . The mechanism of naked DNA uptake and expression. Adv Genet 2005; 54: 3–20.
Heller LC, Heller R . In vivo electroporation for gene therapy. Human Gene Therapy 2006; 17: 890–897.
Favard C, Dean DS, Rols MP . Electrotransfer as a non viral method of gene delivery. Curr Gene Ther 2007; 7: 67–77.
Zhang L, Nolan E, Kreitschitz S, Rabussay DP . Enhanced delivery of naked DNA to the skin by non-invasive in vivo electroporation. Biochimica et Biophysica Acta 2002; 1572: 1–9.
Heller LC, Jaroszeski MJ, Coppola D, Heller R . Comparison of electrically mediated and liposome-complexed plasmid DNA delivery to the skin. Genetic Vaccines and Therapy 2008; 6: 16.
Heller LC, Jaroszeski MJ, Coppola D, McCray AN, Hickey J, Heller R . Optimization of cutaneous electrically mediated plasmid DNA delivery using novel electrode. Gene Therapy 2007; 14: 275–280.
Meykadeh N, Mirmohammadsadegh A, Wang Z, Basner-Tschakarjan E, Hengge UR . Topical application of plasmid DNA to mouse and human skin. J Mol Med 2005; 83: 897–903.
Badea I, Verrall R, Baca-Estrada M, Tikoo S, Rosenberg A, Kumar P et al. In vivo cutaneous interferon-gamma gene delivery using novel dicationic (gemini) surfactant-plasmid complexes. J Gene Med 2005; 7: 1200–1214.
Raghavachari N, Fahl WE . Targeted gene delivery to skin cells in vivo: a comparative study of liposomes and polymers as delivery vehicles. J Pharmaceutical Sci 2002; 91: 615–622.
Shi Z, Curiel DT, Tang DC . DNA-based non-invasive vaccination onto the skin. Vaccine 1999; 17: 2136–2141.
Vyas SP, Singh RP, Jain S, Mishra V, Mahor S, Singh P et al. Non-ionic surfactant based vesicles (niosomes) for non-invasive topical genetic immunization against hepatitis B. Int J Pharm 2005; 296: 80–86.
Wang J, Hu JH, Li FQ, Liu GZ, Zhu QG, Liu JY et al. Strong cellular and humoral immune responses induced by transcutaneous immunization with HBsAg DNA-cationic deformable liposome complex. Exp Dermatol 2007; 16: 724–729.
Ortiz-Urda S, Lin Q, Yant SR, Keene D, Kay MA, Khavari PA . Sustainable correction of junctional epidermolysis bullosa via transposon-mediated nonviral gene transfer. Gene Therapy 2003; 10: 1099–1104.
Ortiz-Urda S, Thyagarajan B, Keene DR, Lin Q, Calos MP, Khavari PA . PhiC31 integrase-mediated nonviral genetic correction of junctional epidermolysis bullosa. Human Gene Therapy 2003; 14: 923–928.
Budker V, Budker T, Zhang G, Subbotin V, Loomis A, Wolff JA . Hypothesis: naked plasmid DNA is taken up by cells in vivo by a receptor-mediated process. J Gene Med 2000; 2: 76–88.
Herweijer H, Wolff JA . Progress and prospects: naked DNA gene transfer and therapy. Gene Therapy 2003; 10: 453–458.
Drabick JJ, Glasspool-Malone J, King A, Malone RW . Cutaneous transfection and immune responses to intradermal nucleic acid vaccination are significantly enhanced by in vivo electropermeabilization. Mol Ther 2001; 3: 249–255.
Gareau DS, Merlino G, Corless C, Kulesz-Martin M, Jacques SL . Noninvasive imaging of melanoma with reflectance mode confocal scanning laser microscopy in a murine model. J Invest Dermatol 2007; 127: 2184–2190.
Li Y, Gonzalez S, Terwey TH, Wolchok J, Aranda I, Toledo-Crow R et al. Dual mode reflectance and fluorescence confocal laser scanning microscopy for in vivo imaging melanoma progression in murine skin. J Invest Dermatol 2005; 125: 798–804.
Ra H, Piyawattanametha W, Mandella MJ, Hsiung PL, Hardy J, Wang TD et al. Three-dimensional in vivo imaging by a handheld dual-axes confocal microscope. Optics Express 2008; 16: 7224–7232.
Gonzalez-Gonzalez E, Ra H, Hickerson RP, Wang Q, Piyawattanametha W, Mandella MJ et al. siRNA silencing of keratinocyte-specific GFP expression in a transgenic mouse skin model. Gene Therapy 2009; 16: 963–972.
Basner-Tschakarjan E, Mirmohammadsadegh A, Baer A, Hengge UR . Uptake and trafficking of DNA in keratinocytes: evidence for DNA-binding proteins. Gene Therapy 2004; 11: 765–774.
Wang Q, Ilves H, Chu P, Contag CH, Leake D, Johnston BH et al. Delivery and inhibition of reporter genes by small interfering RNAs in a mouse skin model. J Invest Dermatol 2007; 127: 2577–2584.
Hengge UR, Chan EF, Foster RA, Walker PS, Vogel JC . Cytokine gene expression in epidermis with biological effects following injection of naked DNA. Nat Genet 1995; 10: 161–166.
Siprashvili Z, Scholl FA, Oliver SF, Adams A, Contag CH, Wender PA et al. Gene transfer via reversible plasmid condensation with cysteine-flanked, internally spaced arginine-rich peptides. Human Gene Therapy 2003; 14: 1225–1233.
Hickerson RP, Smith FJ, Reeves RE, Contag CH, Leake D, Leachman SA et al. Single-nucleotide-specific siRNA targeting in a dominant-negative skin model. J Invest Dermatol 2008; 128: 594–605.
Smith FJ, Hickerson RP, Sayers JM, Reeves RE, Contag CH, Leake D et al. Development of therapeutic siRNAs for pachyonychia congenita. J Invest Dermatol 2008; 128: 50–58.
Kaspar R, McLean W, Schwartz M . Achieving successful delivery of nucleic acids to skin: 6th annual meeting of the international pachyonychia congenita consortium. J Invest Derm 2009; 129: 2085–2087.
Eckstein F . The versatility of oligonucleotides as potential therapeutics. Expert Opin Biol Ther 2007; 7: 1021–1034.
Dorsett Y, Tuschl T . siRNAs: applications in functional genomics and potential as therapeutics. Nat Rev 2004; 3: 318–329.
Scherer LJ, Rossi JJ . Approaches for the sequence-specific knockdown of mRNA. Nat Biotechnol 2003; 21: 1457–1465.
Wraight CJ, White PJ . Antisense oligonucleotides in cutaneous therapy. Pharmacol Therapeut 2001; 90: 89–104.
Geusens B, Sanders N, Prow T, Van Gele M, Lambert J . Cutaneous short-interfering RNA therapy. Expert Opin Drug Deliv 2009; 6: 1333–1349.
Hengge UR, Pfutzner W, Williams M, Goos M, Vogel JC . Efficient expression of naked plasmid DNA in mucosal epithelium: prospective for the treatment of skin lesions. J Invest Dermatol 1998; 111: 605–608.
Budker VG, Subbotin VM, Budker T, Sebestyen MG, Zhang G, Wolff JA . Mechanism of plasmid delivery by hydrodynamic tail vein injection. II. Morphological studies. J Gene Med 2006; 8: 874–888.
Sebestyen MG, Budker VG, Budker T, Subbotin VM, Zhang G, Monahan SD et al. Mechanism of plasmid delivery by hydrodynamic tail vein injection. I. Hepatocyte uptake of various molecules. J Gene Med 2006; 8: 852–873.
Sawamura D, Meng X, Ina S, Ishikawa H, Tamai K, Nomura K et al. In vivo transfer of a foreign gene to keratinocytes using the hemagglutinating virus of Japan-liposome method. J Invest Dermatol 1997; 108: 195–199.
Lin MT, Wang F, Uitto J, Yoon K . Differential expression of tissue-specific promoters by gene gun. Br J Dermatol 2001; 144: 34–39.
Sawamura D, Yasukawa K, Kodama K, Yokota K, Sato-Matsumura KC, Toshihiro T et al. The majority of keratinocytes incorporate intradermally injected plasmid DNA regardless of size but only a small proportion of cells can express the gene product. J Invest Dermatol 2002; 118: 967–971.
Brocard J, Warot X, Wendling O, Messaddeq N, Vonesch JL, Chambon P et al. Spatio-temporally controlled site-specific somatic mutagenesis in the mouse. Proc Natl Acad Sci USA 1997; 94: 14559–14563.
Cao YA, Bachmann MH, Beilhack A, Yang Y, Tanaka M, Swijnenburg RJ et al. Molecular imaging using labeled donor tissues reveals patterns of engraftment, rejection, and survival in transplantation. Transplantation 2005; 80: 134–139.
Vasioukhin V, Degenstein L, Wise B, Fuchs E . The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci USA 1999; 96: 8551–8556.
Sawicki JA, Morris RJ, Monks B, Sakai K, Miyazaki J . A composite CMV-IE enhancer/beta-actin promoter is ubiquitously expressed in mouse cutaneous epithelium. Exp Cell Res 1998; 244: 367–369.
Christophers E, Laurence EB . Regional variations in mouse skin: quantitation of epidermal compartments in two different body sites. Virchows Arch B Cell Pathol 1973; 12: 212–222.
Cameron I . Cell proliferation and renewal in the mammalian body. In: Cameron I, Thrasher J, eds. Cellular And Molecular Renewal in the Mammalian Body. Academic Press: New York, 1971, pp 45–86.
Quinn CM, Wiles AP, El-Shanawany T, Catchpole I, Alnadaf T, Ford MJ et al. The human eukaryotic initiation factor 4AI gene (EIF4A1) contains multiple regulatory elements that direct high-level reporter gene expression in mammalian cell lines. Genomics 1999; 62: 468–476.
Johansen TE, Scholler MS, Tolstoy S, Schwartz TW . Biosynthesis of peptide precursors and protease inhibitors using new constitutive and inducible eukaryotic expression vectors. FEBS Lett 1990; 267: 289–294.
Salmon P, Kindler V, Ducrey O, Chapuis B, Zubler RH, Trono D . High-level transgene expression in human hematopoietic progenitors and differentiated blood lineages after transduction with improved lentiviral vectors. Blood 2000; 96: 3392–3398.
Mikkola H, Woods NB, Sjogren M, Helgadottir H, Hamaguchi I, Jacobsen SE et al. Lentivirus gene transfer in murine hematopoietic progenitor cells is compromised by a delay in proviral integration and results in transduction mosaicism and heterogeneous gene expression in progeny cells. J Virol 2000; 74: 11911–11918.
Contag CH, Bachmann MH . Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng 2002; 4: 235–260.
Acknowledgements
We thank Manny Flores and Heini Ilves for technical support, Tycho Speaker for assistance in data analysis, Cory Nicholas (Stanford University) for providing C57BL/6-Tg(UBC-GFP)30Scha/J and B6;129S-Gt(ROSA)26Sor/J transgenic animals, Jonathan Hardy, Thomas Kledal and Michael Bachmann for providing pEIF4A1 and pTEJ-8 and Soosan Ghazizadeh for providing pHR′EF1–GFP–WPRESIN18 and for useful discussion. This work was supported by NIH Grants R43AR055881-01 and R44AR055881-02 (RLK); and U54 CA105296 (CHC). Emilio Gonzalez is the recipient of a Pachyonychia Congenita Project fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Roger Kaspar, Robyn Hickerson and Ryan Spitler are employees of TransDerm Inc. and Christopher Contag is a founder of Xenogen Corp. now part of Caliper LifeSciences.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
González-González, E., Ra, H., Spitler, R. et al. Increased interstitial pressure improves nucleic acid delivery to skin enabling a comparative analysis of constitutive promoters. Gene Ther 17, 1270–1278 (2010). https://doi.org/10.1038/gt.2010.74
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2010.74
Keywords
This article is cited by
-
Transdermal Delivery of siRNA through Microneedle Array
Scientific Reports (2016)
-
Gene Silencing in Skin After Deposition of Self-Delivery siRNA With a Motorized Microneedle Array Device
Molecular Therapy - Nucleic Acids (2013)
-
Local Delivery of Gene-Modifying Triplex-Forming Molecules to the Epidermis
Journal of Investigative Dermatology (2013)
-
Generic and Personalized RNAi-Based Therapeutics for a Dominant-Negative Epidermal Fragility Disorder
Journal of Investigative Dermatology (2012)
-
DNA Vaccination in the Skin Using Microneedles Improves Protection Against Influenza
Molecular Therapy (2012)