Abstract
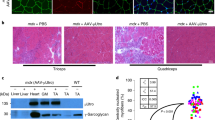
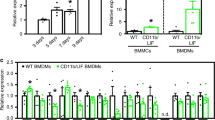
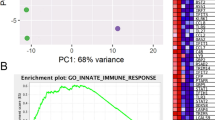
Duchenne muscular dystrophy is a fatal, genetic disorder in which dystrophin-deficient muscle progressively degenerates, for which dystrophin gene transfer could provide effective treatment. The host immune response to dystrophin, however, is an obstacle to therapeutic gene expression. Understanding the dystrophin-induced host immune response will facilitate the discovery of strategies to prolong expression of recombinant dystrophin in dystrophic muscle. Using whole-body irradiation of the dystrophic mdx mouse before gene transfer, we temporally removed the immune system; a 600 rad dose removed peripheral immune cells, which were restored by self-reconstitution, and a 900 rad dose removed central and peripheral immune cells, which were restored by adoptive transfer of bone marrow from a syngeneic, dystrophin-normal donor. The anti-dystrophin humoral response was delayed and dystrophin expression was partially preserved in irradiated, vector-treated mice. Nonirradiated, vector-treated control mice lost muscle dystrophin expression completely, had an earlier anti-dystrophin humoral response and demonstrated muscle fibers focally surrounded with T cells. We conclude that dystrophin gene transfer induced anti-dystrophin humoral immunity and cell-mediated responses that were significantly diminished and delayed by temporal removal of the host central or peripheral immune cells. Furthermore, manipulation of central immunity altered the pattern of regulatory T cells in muscle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Spowart G, Buckton KE, Skinner R, Emery AE . X chromosome in Duchenne muscular dystrophy. Lancet 1982; 1: 1251.
Drousiotou A, Ioannou P, Georgiou T, Mavrikiou E, Christopoulos G, Kyriakides T et al. Neonatal screening for Duchenne muscular dystrophy: a novel semiquantitative application of the bioluminescence test for creatine kinase in a pilot national program in Cyprus. Genet Test 1998; 2: 55–60.
Emery AE . Population frequencies of inherited neuromuscular diseases—a world survey. Neuromuscul Disord 1991; 1: 19–29.
Parsons EP, Bradley DM, Clarke AJ . Newborn screening for Duchenne muscular dystrophy. Arch Dis Child 2003; 88: 91–92.
Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H, Kunkel LM . An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics 1988; 2: 90–95.
Hoffman EP, Brown Jr RH, Kunkel LM . Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987; 51: 919–928.
Love DR, Davies KE . Duchenne muscular dystrophy: the gene and the protein. Mol Biol Med 1989; 6: 7–17.
Koenig M, Monaco AP, Kunkel LM . The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell 1988; 53: 219–228.
Groh S, Zong H, Goddeeris MM, Lebakken CS, Venzke D, Pessin JE et al. Sarcoglycan complex: implications for metabolic defects in muscular dystrophies. J Biol Chem 2009; 284: 19178–19182.
Satz JS, Philp AR, Nguyen H, Kusano H, Lee J, Turk R et al. Visual impairment in the absence of dystroglycan. J Neurosci 2009; 29: 13136–13146.
Yoshida M, Ozawa E . Glycoprotein complex anchoring dystrophin to sarcolemma. J Biochem 1990; 108: 748–752.
Bonilla E, Samitt CE, Miranda AF, Hays AP, Salviati G, DiMauro S et al. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell 1988; 54: 447–452.
Emery AE . Dystrophin function. Lancet 1990; 335: 1289.
Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ . The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 1989; 244: 1578–1580.
Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, Kota J, Coley BD, Galloway G et al. Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol 2009; 66: 290–297.
Gilbert R, Nalbantoglu J, Howell JM, Davies L, Fletcher S, Amalfitano A et al. Dystrophin expression in muscle following gene transfer with a fully deleted (‘gutted’) adenovirus is markedly improved by trans-acting adenoviral gene products. Hum Gene Ther 2001; 12: 1741–1755.
Alba R, Bosch A, Chillon M . Gutless adenovirus: last-generation adenovirus for gene therapy. Gene Therapy 2005; 12: 18–27.
Jiang Z, Schiedner G, Gilchrist S, Kochanek S, Clemens PR . CTLA4Ig delivered by high-capacity adenoviral vector induces stable expression of dystrophin in mdx mouse muscle. Gene Therapy 2007; 11: 1453–1461.
Acsadi G, Lochmuller H, Jani A, Huard J, Massie B, Prescott S et al. Dystrophin expression in muscles of mdx mice after adenovirus-mediated in vivo gene transfer. Hum Gene Ther 1996; 7: 129–140.
Ferrer A, Wells KE, Wells DJ . Immune responses to dystropin: implications for gene therapy of Duchenne muscular dystrophy. Gene Ther 2000; 7: 1439–1446.
Gilchrist S, Ontel M, Kochanek S, Clemens PR . Immune response to full-length dystrophin delivered to Dmd muscle by a high-capacity adenoviral vector. Mol Ther 2002; 6: 359–368.
Danko I, Chapman V, Wolff JA . The frequency of revertants in mdx mouse genetic models for Duchenne muscular dystrophy. Pediatr Res 1992; 32: 128–131.
Hoffman EP, Morgan JE, Watkins SC, Partridge TA . Somatic reversion/suppression of the mouse mdx phenotype in vivo. J Neurol Sci 1990; 99: 9–25.
Sahashi K, Ibi T, Suoh H, Nakao N, Tashiro M, Marui K et al. Immunostaining of dystrophin and utrophin in skeletal muscle of dystrophinopathies. Intern Med 1994; 33: 277–283.
Thanh LT, Nguyen TM, Helliwell TR, Morris GE . Characterization of revertant muscle fibers in Duchenne muscular dystrophy, using exon-specific monoclonal antibodies against dystrophin. Am J Hum Genet 1995; 56: 725–731.
Appleby MW, Ramsdell F . Scurfy, the Foxp3 locus, and the molecular basis of peripheral tolerance. Curr Top Microbiol Immunol 2008; 321: 151–168.
Sakaguchi S . Regulatory T cells: key controllers of immunologic self-tolerance. Cell 2000; 101: 455–458.
Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev 2006; 212: 8–27.
Sakaguchi S, Yamaguchi T, Nomura T, Ono M . Regulatory T cells and immune tolerance. Cell 2008; 133: 775–787.
Eghtesad S, Morel PA, Clemens PR . The companions: regulatory T cells and gene therapy. Immunology 2009; 127: 1–7.
Spencer MJ, Tidball JG . Do immune cells promote the pathology of dystrophin-deficient myopathies? Neuromuscul Disord 2001; 11: 556–564.
Fontenot JD, Gavin MA, Rudensky AY . Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4: 330–336.
Yagi H, Nomura T, Kakamura K, Yamazaki S, Kitawaki T, Hori S et al. Crucial role of Foxp3 in the development and function of human CD4+CD25+ regulatory T cells. Jpn Soc Immunol 2004; 16: 1643–1656.
Cheng X, Dai H, Wan N, Moore Y, Vankayalapati R, Dai Z . Interaction of programmed death-1 and programmed death-1 ligand-1 contributes to testicular immune privilege. Transplantation 2009; 87: 1778–1786.
Dai H, Zhu H, Lei P, Yagita H, Liu J, Wen X et al. Programmed death-1 signaling is essential for the skin allograft protection by alternatively activated dendritic cell infusion in mice. Transplantation 2009; 88: 864–873.
Folkl A, Bienzle D . Structure and function of programmed death (PD) molecules. Vet Immunol Immunopathol 2010; 134: 33–38.
Hagiwara H, Ohsawa Y, Asakura S, Murakami T, Teshima T, Sunada Y . Bone marrow transplantation improves outcome in a mouse model of congenital muscular dystrophy. FEBS Lett 2006; 580: 4463–4468.
Camirand G, Stephan L, Rousseau J, Sackett MK, Caron NJ, Mills P et al. Central tolerance to myogenic cell transplants does not include muscle neoantigens. Transplantation 2008; 85: 1791–1801.
Feng SW, Lu XL, Liu ZS, Zhang YN, Liu TY, Li JL et al. Dynamic distribution of bone marrow-derived mesenchymal stromal cells and change of pathology after infusing into mdx mice. Cytotherapy 2008; 10: 254–264.
Camirand G, Stephan L, Rousseau J, Sackett MK, Caron NJ, Mills P et al. Central tolerance to myogenic cell transplants does not include muscle neoantigens. Transplantation 2008; 85: 1791–1801.
Quinlan JG, Lyden SP, Cambier DM, Johnson SR, Michaels SE, Denman DL . Radiation inhibition of mdx mouse muscle regeneration: dose and age factors. Muscle Nerve 1995; 18: 201–206.
Chretien F, Dreyfus PA, Christov C, Caramelle P, Lagrange JL, Chazaud B et al. In vivo fusion of circulating fluorescent cells with dystrophin-deficient myofibers results in extensive sarcoplasmic fluorescence expression but limited dystrophin sarcolemmal expression. Am J Pathol 2005; 166: 1741–1748.
Spencer MJ, Montecino-Rodriguez E, Dorshkind K, Tidball JG . Helper (CD4(+)) and cytotoxic (CD8(+)) T cells promote the pathology of dystrophin-deficient muscle. Clin Immunol 2001; 98: 235–243.
Jiang Z, Schiedner G, van Rooijen N, Liu CC, Kochanek S, Clemens PR . Sustained muscle expression of dystrophin from a high-capacity adenoviral vector with systemic gene transfer of T cell costimulatory blockade. Mol Ther 2004; 10: 688–696.
Farini A, Meregalli M, Belicchi M, Battistelli M, Parolini D, D’Antona G et al. T and B lymphocyte depletion has a marked effect on the fibrosis of dystrophic skeletal muscles in the scid/mdx mouse. J Pathol 2007; 213: 229–238.
Camirand G, Stephan L, Rousseau J, Sackett MK, Caron NJ, Mills P et al. Central tolerance to myogenic cell transplants does not include muscle neoantigens. Transplantation 2008; 85: 1791–1801.
Jiang Z, Schiedner G, van Rooijen N, Liu CC, Kochanek S, Clemens PR . Sustained muscle expression of dystrophin from a high-capacity adenoviral vector with systemic gene transfer of T cell costimulatory blockade. Mol Ther 2004; 10: 688–696.
Acknowledgements
We thank Daniel P Reay for providing valuable technical support and breeding mice used in these studies. We also thank Penelope A Morel for her constructive critique of this paper. This work was supported by F31-NS056780-01A2 (SE) from the NIH and Grant W81XWH-05-1-0334 (PRC) from the US Army Medical Research and Materiel Command. The authors take full responsibility for the contents of this paper, which do not represent the views of the Department of Veterans Affairs or the United States Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Eghtesad, S., Zheng, H., Nakai, H. et al. Effects of irradiating adult mdx mice before full-length dystrophin cDNA transfer on host anti-dystrophin immunity. Gene Ther 17, 1181–1190 (2010). https://doi.org/10.1038/gt.2010.108
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2010.108
Keywords
This article is cited by
-
Effect of rapamycin on immunity induced by vector-mediated dystrophin expression in mdx skeletal muscle
Scientific Reports (2012)