Abstract
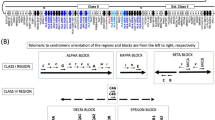
The human FCGR2/3 locus, containing five highly homologous genes encoding the major IgG receptors, shows extensive copy number variation (CNV) associated with susceptibility to autoimmune diseases. Having genotyped >4000 individuals, we show that all CNV at this locus can be explained by nonallelic homologous recombination (NAHR) of the two paralogous repeats that constitute the majority of the locus, and describe four distinct CNV regions (CNRs) with a highly variable prevalence in the population. Apart from CNV, NAHR events also created several hitherto unidentified chimeric FCGR2 genes. These include an FCGR2A/2C chimeric gene that causes a decreased expression of FcγRIIa on phagocytes, resulting in a decreased production of reactive oxygen species in response to immune complexes, compared with wild-type FCGR2A. Conversely, FCGR2C/2A chimeric genes were identified to lead to an increased expression of FCGR2C. Finally, a rare FCGR2B null-variant allele was found, in which a polymorphic stop codon of FCGR2C is introduced into one FCGR2B gene, resulting in a 50% reduction in protein expression. Our study on CNRs and the chimeric genes is essential for the correct interpretation of association studies on FCGR genes as a determinant for disease susceptibility, and may explain some as yet unidentified extreme phenotypes of immune-mediated disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bruhns P . Properties of mouse and human IgG receptors and their contribution to disease models. Blood 2012; 119: 5640–5649.
Nagelkerke SQ, Kuijpers TW . Immunomodulation by IVIg and the role of Fc-gamma receptors: classic mechanisms of action after all? Front Immunol 2014; 5: 674.
Breunis WB, van Mirre E, Bruin M, Geissler J, de Boer M, Peters M et al. Copy number variation of the activating FCGR2C gene predisposes to idiopathic thrombocytopenic purpura. Blood 2008; 111: 1029–1038.
Fanciulli M, Norsworthy PJ, Petretto E, Dong R, Harper L, Kamesh L et al. FCGR3B copy number variation is associated with susceptibility to systemic, but not organ-specific, autoimmunity. Nat Genet 2007; 39: 721–723.
Khor CC, Davila S, Breunis WB, Lee YC, Shimizu C, Wright VJ et al. Genome-wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nat Genet 2011; 43: 1241–1246.
Niederer HA, Willcocks LC, Rayner TF, Yang W, Lau YL, Williams TN et al. Copy number, linkage disequilibrium and disease association in the FCGR locus. Hum Mol Genet 2010; 19: 3282–3294.
Mueller M, Barros P, Witherden AS, Roberts AL, Zhang Z, Schaschl H et al. Genomic pathology of SLE-associated copy-number variation at the FCGR2C/FCGR3B/FCGR2B locus. Am J Hum Genet 2013; 92: 28–40.
Asano K, Matsushita T, Umeno J, Hosono N, Takahashi A, Kawaguchi T et al. A genome-wide association study identifies three new susceptibility loci for ulcerative colitis in the Japanese population. Nat Genet 2009; 41: 1325–1329.
Lee YH, Ji JD, Song GG . Fcgamma receptor IIB and IIIB polymorphisms and susceptibility to systemic lupus erythematosus and lupus nephritis: a meta-analysis. Lupus 2009; 18: 727–734.
Chai L, Song YQ, Leung WK . Genetic polymorphism studies in periodontitis and Fcgamma receptors. J Periodontal Res 2012; 47: 273–285.
Adu B, Dodoo D, Adukpo S, Hedley PL, Arthur FK, Gerds TA et al. Fc gamma receptor IIIB (FcgammaRIIIB) polymorphisms are associated with clinical malaria in ghanaian children. PLoS One 2012; 7: e46197.
Mellor JD, Brown MP, Irving HR, Zalcberg JR, Dobrovic A . A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J Hematol Oncol 2013; 6: 1.
Tamura K, Shimizu C, Hojo T, Akashi-Tanaka S, Kinoshita T, Yonemori K et al. FcgammaR2A and 3A polymorphisms predict clinical outcome of trastuzumab in both neoadjuvant and metastatic settings in patients with HER2-positive breast cancer. Ann Oncol 2011; 22: 1302–1307.
Warmerdam PA, Nabben NM, van de Graaf SA, van de Winkel JG, Capel PJ . The human low affinity immunoglobulin G Fc receptor IIC gene is a result of an unequal crossover event. J Biol Chem 1993; 268: 7346–7349.
van der Heijden J, Breunis WB, Geissler J, de Boer M, van den Berg TK, Kuijpers TW . Phenotypic variation in IgG receptors by nonclassical FCGR2C alleles. J Immunol 2012; 188: 1318–1324.
Breunis WB, van Mirre E, Geissler J, Laddach N, Wolbink G, van der Schoot E et al. Copy number variation at the FCGR locus includes FCGR3A, FCGR2C and FCGR3B but not FCGR2A and FCGR2B. Hum Mutat 2009; 30: E640–E650.
Machado LR, Hardwick RJ, Bowdrey J, Bogle H, Knowles TJ, Sironi M et al. Evolutionary history of copy-number-variable locus for the low-affinity Fcgamma receptor: mutation rate, autoimmune disease, and the legacy of helminth infection. Am J Hum Genet 2012; 90: 973–985.
Emanuel BS, Shaikh TH . Segmental duplications: an 'expanding' role in genomic instability and disease. Nat Rev Genet 2001; 2: 791–800.
Su K, Wu J, Edberg JC, McKenzie SE, Kimberly RP . Genomic organization of classical human low-affinity Fcgamma receptor genes. Genes Immun 2002; 3: S51–S56.
Chen JY, Wang CM, Chang SW, Cheng CH, Wu YJ, Lin JC et al. Association of FCGR3A and FCGR3B copy number variations with systemic lupus erythematosus and rheumatoid arthritis in Taiwanese patients. Arthritis Rheumatol 2014; 66: 3113–3121.
Boross P, Arandhara VL, Martin-Ramirez J, Santiago-Raber ML, Carlucci F, Flierman R et al. The inhibiting Fc receptor for IgG, FcgammaRIIB, is a modifier of autoimmune susceptibility. J Immunol 2011; 187: 1304–1313.
Espeli M, Niederer HA, Traherne JA, Trowsdale J, Smith KG . Genetic variation, Fcgamma receptors, KIRs and infection: the evolution of autoimmunity. Curr Opin Immunol 2010; 22: 715–722.
Li H . Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM 2013; 00: 1–3 arXiv:1303 3997v2 [q-bio GN].
DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 2011; 43: 491–498.
van der Heijden J, Nagelkerke S, Zhao X, Geissler J, Rispens T, van den Berg TK et al. Haplotypes of FcgammaRIIa and FcgammaRIIIb polymorphic variants influence IgG-mediated responses in neutrophils. J Immunol 2014; 192: 2715–2721.
Acknowledgements
We thank Dr Marc Bijl, Mr Michel Tsang-a-Sjoe, Professor Dr Alexandre Voskuijl, Dr Bart Biemond, Mrs Lisa Vogelpoel and Professor Dr Martin Hibberd for help in sample collection, and Professor Dr Frank Baas for help with data analysis. This work was supported by a grant from the Landsteiner Foundation for Blood transfusion Research (LSBR 0916). CET has received a grant of the Ter Meulen Fund, Royal Netherlands Academy of Arts and Sciences (TMF2012/227).
Author Contributions
SQN performed experiments, analyzed data, wrote the manuscript and designed the research; CET and JG performed experiments and analyzed data; WBB discussed data and designed the research; JWRS provided samples; BA analyzed data; TKB discussed data; MB performed experiments, analyzed and discussed data, and designed the research; and TWK discussed data, wrote the manuscript and designed the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Genes and Immunity website
Supplementary information
Rights and permissions
About this article
Cite this article
Nagelkerke, S., Tacke, C., Breunis, W. et al. Nonallelic homologous recombination of the FCGR2/3 locus results in copy number variation and novel chimeric FCGR2 genes with aberrant functional expression. Genes Immun 16, 422–429 (2015). https://doi.org/10.1038/gene.2015.25
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gene.2015.25
This article is cited by
-
Fcγ receptors and immunomodulatory antibodies in cancer
Nature Reviews Cancer (2024)
-
FCGR3A and FCGR3B copy number variations are risk factors for sarcoidosis
Human Genetics (2016)