Abstract
Purpose
International variations in visual acuity (VA) outcomes of eyes treated for neovascular age-related macular degeneration (nAMD) are well-documented, but intra-country inter-centre regional variations are not known. These data are important for national quality outcome indicators. We aimed to determine intra-country and inter-centre regional variations in outcomes for treatment of nAMD.
Patients and methods
Prospective multicentre national database study of 13 UK centres that treated patients according to a set protocol (three loading doses, followed by Pro-Re-Nata retreatment). A total of 5811 treatment naive eyes of 5205 patients received a total of 36 206 ranibizumab injections over 12 months.
Results
Mean starting VA between centres varied from 48.9 to 59.9 ETDRS letters. Mean inter-centre VA change from baseline to 12 months varied from +6.9 letters to −0.6 letters (mean of +2.5 letters). The proportion of eyes achieving VA of 70 letters or more varied between 21.9 and 48.7% at 12 months. Median number of injections (visits) at each centre varied from 5 to 8 (9 to 12), with an overall median of 6 (11). Age, starting VA, number of injections, and visits, but not gender were significantly associated with variation in these VA outcomes (P<0.01). Significant variation between centres persisted even after adjusting for these factors.
Conclusion
There are modest differences in VA outcomes between centres in the UK. These differences are influenced, but not completely explained, by factors such as patient age, starting VA, number of injections, and visits. These data provide an indication of the VA outcomes that are achievable in real-world settings.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is one of the leading causes of irreversible blindness in patients aged over 60 years. Since the introduction of anti-vascular endothelial growth factor agents to treat neovascular AMD (nAMD) in 2006, rates of blindness and visual impairment from AMD have declined markedly.1, 2, 3 Data from several randomised clinical trials suggest patients gain on average 6–11 letters using the most aggressive monthly dosing posology in the first year of treatment.4, 5, 6, 7 A less resource intensive Pro-Re-Nata (PRN)-dosing posology has been found in the Comparison of Age-related macular degeneration Treatment Trials (CATT)6, 8 and Inhibit VEGF in Age-related choroidal Neovascularisation (IVAN)7, 9 trials to produce outcomes similar to monthly dosing. This has provided a sound empirical basis for use of PRN posology in the National Health System (NHS) in the UK.
Although PRN-dosing posology in theory is able to achieve excellent outcomes, ‘real-world’ studies have not matched the outcomes achieved in clinical trials.10, 11, 12, 13 We have previously reported longitudinal results from a cohort of patients recruited from hospitals in the UK.10, 11, 14 Patients had standardised data recorded at the point of care into an electronic medical record (EMR) system, and the grouped national findings showed that PRN retreatment after three loading doses resulted in moderately stable vision.11 National data have also been reported from Australia for a treat and extend posology.15 International multi-country real life comparisons have reported some international differences in VA outcomes and treatment patterns,16which may be influenced by different reimbursement and health system structures. However, what remains unclear is how much, if any, intra-country inter-centre regional variation occurs. These data are obscured by aggregate national data, and is important, as it permits the understanding of the factors that influence outcomes in a real-world setting and allows national quality benchmarks to be set. In this report, we examine inter-centre variations in patient characteristics, the number of treatments delivered, and their impact on visual outcomes in 13 geographically distinct UK centres.
Materials and methods
Study design
The study design is described in detail in previous reports from the UK-nAMD Database Study group.10, 11, 14 In brief, sites known to make comprehensive use of EMR systems were contacted and requested to contribute data. Patient identifiers were completely removed, and site and clinician data were pseudo-anonymised. On this basis, an ethics committee determined that formal ethics approval was not required. The study was conducted in accordance with the declaration of Helsinki and the UK’s Data Protection Act.
Study centres
Thirteen NHS hospitals that deliver ranibizumab AMD treatment services in England and Northern Ireland submitted data to this study. Each site is the sole NHS provider of nAMD care to their local population and very few patients switch between providers. Following NICE approval for the use of ranibizumab for nAMD in the NHS in August 2008, all sites used this drug almost exclusively, although prior to this date, some sites offered limited treatment with bevacizumab. The study was initiated on 1 February 2012, all approvals and data extraction were performed by 2 April 2012. Data were delivered to the analysis team by the end of April 2012.
Data variables
Analysis was restricted to treatment-naive eyes undergoing ranibizumab therapy conforming to three monthly loading doses followed by PRN posology for nAMD that completed at least 12 months of follow-up.
In this report, the ‘best-measured VA’ was the best VA with refraction or habitual correction and/or pinhole as measured on an ETDRS chart, and expressed as LogMAR vision and ETDRS letters. The vast majority of sites measured VA with habitual correction rather than best-corrected refracted VA at all time points and used ETDRS charts. Analysis for eyes with very low VA was undertaken by substituting counting fingers, hand movement, and perception of light with 2.0, 2.3, and 2.7, respectively.
We examined the outcome measures commonly reported in clinical trials (VA change from 0 to 12 months, proportion of eyes gaining and losing 15 ETDRS letters, etc), as well as other measures such as VA change from 3 to 12 months and proportion of eyes achieving 70 ETDRS letters at 12 months (Snellen equivalent of 20/40 or 6/12, driving equivalent in many jurisdictions).
Statistical methods
Medisoft Ophthalmology (Medisoft Limited, Leeds, UK) was the EMR system used for data extraction. Data for right and left eyes of patients who had had at least one intravitreal injection of ranibizumab for nAMD were extracted. Both STATA version 11 (Statacorp LP, College Station, TX, USA) and SPSS version 19 (IBM, Armonk, NY, USA) were used to analyse data. Perl and R package ggplot2 (R-Project, free software) was used for multivariable analyses, construction of generalised linear models, and creation of funnel plots.
Results
Participants
Over the 1 year of follow-up analysed in this study, 36 206 ranibizumab injections were performed in 5811 eyes. Table 1 shows the baseline demographics of each of the 13 centres. The number of eyes treated ranged from 39 (centre M) to 923 (centre C). Patients treated at each centre were of similar age, ranging from a mean of 78.3 (J) to 81.7 years at the time of the first injection (K).
Treatment and outcome characteristics of each centre are shown in Table 2. The median number of injections in each centre was 6, with one centre providing a median of 8 injections (J) and two centres providing a median of 5 (L, M). Mean starting VA across all centres was lowest in centre F (48.9 ETDRS letters) and highest in centre L (59.9 letters). VA after 12 months of treatment was highest in centre I (63.2 letters) and lowest in centre M (52.9 letters). The centres with the higher mean starting VA did not necessarily finish 12 months with the highest VA at 1 year, nor did the centres with the lowest starting VA finish with the lowest VA at 1 year. Centres that saw patients most frequently (B) or injected most frequently (J) achieved the fifth and second best VA at 1 year, respectively, and showed the least variation between 3 and 12 months.
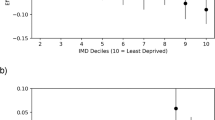
At 12 months, the mean change in VA across 13 centres varied from +6.9 letters (centre I) to −0.6 letters (centre L), a difference of 7.5 letters, with a mean VA gain of +2.5 letters. Figure 1 shows these results graphically, with further details in Table 2. The funnel plot in Figure 1 shows the distribution of centres, and 95 and 99% confidence intervals (CIs). Two centres (L, D) were slightly outside the 99% CIs; these were the only centres reporting a slight reduction in VA. One of these centres had the lowest number of injections (five, L); however, the other centre had the median number of injections (six, D). Starting VA in centre D was near the middle of the distribution, at 53.7 letters, whereas in centre L, eyes commenced treatment with the best starting VA at 59.9 letters. In contrast, Supplementary Figure 1 shows that there was less variation in VA change from 0 to 3 months, with a tighter clustering of results. Supplementary Figure 2 shows the VA change from 3 to 12 months, with most centres clustered within the 95% CIs, and the previous two centres that were outside the 99% CI when considering change from 0 to 12 months are now either within or very close to the limits (L, D).
The proportion of eyes that gained 15 ETDRS letters or more at 12 months ranged from 7.7 (A) to 29.5% (K). When examined on the funnel plot (Supplementary Figure 3), these proportions showed little variation from centre to centre, with all centres within or above the 99% CI. The proportion of eyes that lost 15 ETDRS letters or more at 12 months showed an even tighter distribution, with all centres within the 99% CI, and all except one (L) within the 95% CI (Supplementary Figure 4). The actual proportions ranged from 4.6 (I) to 11.7% (L). We also examined the proportion of eyes maintaining or achieving driving vision of 70 ETDRS letters or more, and found all centres performed above the lower 99% CI limit (Figure 2).
It should be noted that the centre with the lowest number of visits and injections (M, median 9 visits and median 5 injections, Table 2) had outcomes in the middle of the distributions for all the VA measures studied (Figures 1 and 2). Similarly, the centres with the highest number of visits (B, 12 visits) and highest number of injections (J, 8 injections), generally had outcomes either in the middle of the distribution or higher than average, but still within the 95% CI (Figures 1 and 2).
We performed multivariable analyses to determine which factors were associated with better visual outcomes. Younger age, worse starting VA, and higher number of injections and visits, but not gender were significantly associated with variation in these VA outcomes (P<0.01). Significant variation between centres persisted even after adjusting for these factors.
Discussion
There is considerable published data on VA outcomes derived from clinical trials, but limited data describing real-world outcomes. Real-world outcomes indicating what is possible when trial results translate to clinical practice are ultimately the most important measure, as they reflect what happens to whole populations of patients rather than the rarefied cohorts included in the trials. They are also important for establishing benchmark standards that are achievable in busy public systems, and for defining measures of quality care that take into account the heterogeneity of patient populations and care delivery systems. This study provides some of the first real-world outcomes from a single national health system, namely 13 UK public hospital centres using a PRN treatment posology. We report that there was some inter-centre variation in VA outcomes up to a maximum difference of 7.5 letters between the highest and lowest VA achieved from 0 to 12 months. Age, starting VA, number of injections, and visits, but not gender were significantly associated with variation in these VA outcomes.
The median performance of these 13 centres is comparable with results from clinical trials, once the lower starting VA and lower number of injections are taken into account. Table 3 compares findings from this study with clinical trial results. The CATT6 and IVAN7, 9 studies achieved mean improvement from 0 to 12 months of 6.8 and 5.0 letters, respectively, with seven injections, whereas the Groupe d’Etude Français Avastin versus Lucentis dans la DMLA néovasculaire (GEFAL)17 and Multicentre Anti-VEGF Trial in Austria (MANTA)18 studies showed mean improvement of 2.9 and 4.1 letters, respectively, with six injections, which is comparable to the mean improvement of 2.5 letters with six injections in this study. These results suggest that a similar benchmark of +2.5 letters improvement (0–12 months) with six injections represents quality ongoing care that is achievable in a real-world, public hospital setting.
There are few other real-world studies with which to compare our results. A large database observational study from Australia, the Fight Retinal Blindness Study,19 reported mean VA gains of 5.3 letters after 2 years, and 13 injections of a treat and extend posology. These results are superior to those achieved in this report, but direct comparisons are difficult due to differences in health systems, patient mix, and different follow-up periods.
It should be noted that the difference in 0–12 month VA change between the highest and lowest scoring centres was 7.5 ETDRS letters, a difference that is only marginally beyond what some studies have considered noninferior. The CATT6 and GEFAL17 considered a difference of five EDTRS letters to represent noninferiority, whereas the MANTA18 considered a difference of seven letters to be noninferior.18
Because of the ‘ceiling effect’, whereby eyes starting treatment with good VA have little room for further improvement, many measures are dependent on the starting VA.11, 12, 20 Adjusting for age, starting VA, number of injections, and visits reduced, but did not eliminate the significant variation between centres, suggesting there are other unmeasured factors that contribute to these variations in outcomes.
This study has several strengths including a large sequential sample, collection of a standardised minimum data set as mandated by the use of an EMR reflecting routine, real-world clinical practice, and the large number of centres involved. A weakness of this study is the loss to follow-up of a number of patients over time, as is inevitable in a real-world clinical setting. Although there were differences in baseline demographics between patients lost to follow-up and those who completed follow-up, we have previously shown that VA changes are similar in both groups.11 Best-corrected VAs were not routinely measured, instead the VAs with habitual correction were reported in this study, which may underestimate absolute VA measurements compared with clinical trials. It should be noted that clinical treatment decisions were based on these VAs, and we believe these represent real-world outcomes and may better reflect patients’ visual experience than protocol-determined best-corrected VAs. A possible reason for differences in injection numbers may be individual differences in centre/physician thresholds for retreatment using a PRN posology.
In summary, we report that 13 UK centres using a PRN treatment posology for managing nAMD achieved broadly similar VA outcomes with modest variability in outcomes. The difference between the highest and lowest VA gain at 12 months was 7.5 letters with a mean of +2.5 letters gained. Age, starting VA, number of injections, and visits, but not gender were significantly associated with variation in these VA outcomes. These data may be considered as establishing an achievable benchmark for the quality of PRN posology in real-world settings, and is likely to be relevant to many sites worldwide.

References
Bloch SB, Larsen M, Munch IC . Incidence of legal blindness from age-related macular degeneration in Denmark: year 2000 to 2010. Am J Ophthalmol 2012; 153: 209–213.
Skaat A, Chetrit A, Belkin M, Kinori M, Kalter-Leibovici O . Time trends in the incidence and causes of blindness in Israel. Am J Ophthalmol 2012; 153: 214–221.
Buckle M, Donachie PH, Johnston RL . Long-term outcomes of intravitreal ranibizumab for neovascular age-related macular degeneration in a well defined region of the UK. Br J Ophthalmol 2015; 100: 240–245.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1419–1431.
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1432–1444.
Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ . Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011; 364: 1897–1908.
Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 2012; 119: 1399–1411.
Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012; 119: 1388–1398.
Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet 2013; 382: 1258–1267.
Zarranz-Ventura J, Liew G, Johnston RL, Xing W, Akerele T, McKibbin M et al. The neovascular age-related macular degeneration database: report 2: incidence, management, and visual outcomes of second treated eyes. Ophthalmology 2014; 121: 1966–1975.
Writing Committee for the UK Age-Related Macular Degeneration EMR Users Group. The neovascular age-related macular degeneration database: multicentre study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology 2014; 121: 1092–1101.
Gabai A, Veritti D, Lanzetta P . One-year outcome of ranibizumab for neovascular age-related macular degeneration: a thorough analysis in a real-world clinical setting. Eur J Ophthalmol 2014; 24: 396–401.
Dadgostar H, Ventura AA, Chung JY, Sharma S, Kaiser PK . Evaluation of injection frequency and visual acuity outcomes for ranibizumab monotherapy in exudative age-related macular degeneration. Ophthalmology 2009; 116: 1740–1747.
Lee AY, Lee CS, Butt T, Xing W, Johnston RL, Chakravarthy U et al. UK AMD EMR USERS GROUP REPORT V: benefits of initiating ranibizumab therapy for neovascular AMD in eyes with vision better than 6/12. Br J Ophthalmol 2015; 99: 1045–1050.
Gillies MC, Walton R, Simpson JM, Arnold JJ, Guymer RH, McAllister IL et al. Prospective audit of exudative age-related macular degeneration: 12-month outcomes in treatment-naive eyes. Invest Ophthalmol Vis Sci 2013; 54: 5754–5760.
Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Cortez R et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol 2015; 99: 220–226.
Kodjikian L, Souied EH, Mimoun G, Mauget-Faysse M, Behar-Cohen F, Decullier E et al. Ranibizumab versus bevacizumab for neovascular age-related macular degeneration: results from the GEFAL Noninferiority Randomized Trial. Ophthalmology 2013; 120: 2300–2309.
Krebs I, Schmetterer L, Boltz A, Told R, Vecsei-Marlovits V, Egger S et al. A randomised double-masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age-related macular degeneration. Br J Ophthalmol 2013; 97: 266–271.
Arnold JJ, Campain A, Barthelmes D, Simpson JM, Guymer RH, Hunyor AP et al. Two-year outcomes of "treat and extend" intravitreal therapy for neovascular age-related macular degeneration. Ophthalmology 2015; 122: 1212–1219.
Ross AH, Donachie PH, Sallam A, Stratton IM, Mohamed Q, Scanlon PH et al. Which visual acuity measurements define high-quality care for patients with neovascular age-related macular degeneration treated with ranibizumab? Eye (Lond) 2013; 27: 56–64.
Acknowledgements
This study group has received a proportion of its funding from the Department of Health’s NIHR Biomedical Research Centre for Ophthalmology at Moorfields Eye Hospital and UCL Institute of Ophthalmology. CSL is funded by NIH/NEI grant NIH/NEI K23EY024921. The views expressed in the publication are those of the authors and not necessarily those of the Department of Health. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. We acknowledge Paul Donachie, Gloucestershire Hospitals NHS Foundation Trust, for providing statistical advice on the manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
RLJ is the medical director of Medisoft Limited, the Electronic Medical Record software provider from which data were extracted. The remaining authors declare no conflict of interest.
Additional information
GL and AF had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
The members of the UK AMD EMR Users Group (listed by centre and within each centre)
G Liew1,17, AY Lee2, J Zarranz-Ventura3, I Stratton3, C Bunce1, U Chakravarthy4, CS Lee2, PA Keane1, DA Sim1, T Akerele5, M McKibbin6, L Downey7, S Natha8, C Bailey9, R Khan10, R Antcliff11, S Armstrong12, A Varma13, V Kumar14, M Tsaloumas15, K Mandal16, C Egan1, RL Johnston3, A Tufail1, Q Mohamed3, A Sallam3, P Donachie3, S Arora7, H Cook7, K Gopalakrishnan7, F Lyon7, T Islam7, N Zaman7, O Backhouse6, T Dabbs6, B Davies6, B Mokete6, D O’Neill6, PJ Patel1, W Xing1
1Moorfields Eye Hospital NHS Trust, London, UK; 2University of Washington, WA, USA; 3Gloucestershire Hospitals NHS Foundation Trust, UK; 4Belfast Health and Social Care Trust, UK; 5Hinchingbrooke Health Care NHS Trust, UK; 6Leeds Teaching Hospitals NHS Trust, UK; 7Hull and East Yorkshire Hospitals NHS Trust, UK; 8Wrightington, Wigan and Leigh NHS Foundation Trust, UK; 9University Hospitals Bristol NHS Foundation Trust, UK; 10Calderdale and Huddersfield NHS Foundation Trust, UK; 11Royal United Hospital Bath NHS Trust, UK; 12Countess of Chester Hospital NHS Trust, UK; 13Mid Yorkshire Hospitals NHS Trust, UK; 14Wirral University Teaching Hospital NHS Foundation Trust, UK; 15University Hospital Birmingham NHS Foundation Trust, UK; 16Warrington and Halton Hospitals NHS Foundation Trust, UK; 17University of Sydney, Sydney, New South Wales, Australia.
Supplementary Information accompanies this paper on Eye website
Rights and permissions
About this article
Cite this article
Liew, G., Lee, A., Zarranz-Ventura, J. et al. The UK Neovascular AMD Database Report 3: inter-centre variation in visual acuity outcomes and establishing real-world measures of care. Eye 30, 1462–1468 (2016). https://doi.org/10.1038/eye.2016.149
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2016.149
This article is cited by
-
Long-term outcomes of intravitreal anti-VEGF therapies in patients affected by neovascular age-related macular degeneration: a real-life study
BMC Ophthalmology (2021)
-
Demographic and Clinical Factors that Influence the Visual Response to Anti-Vascular Endothelial Growth Factor Therapy in Patients with Neovascular Age-Related Macular Degeneration: A Systematic Review
Ophthalmology and Therapy (2020)
-
Action on neovascular age-related macular degeneration (nAMD): recommendations for management and service provision in the UK hospital eye service
Eye (2019)
-
Appropriateness of quality standards for meaningful intercentre comparisons of aflibercept service provision for neovascular age-related macular degeneration
Eye (2017)
-
Variations in Treatment Delivery for Patients with Neovascular AMD in the UK: Results from an Ophthalmology Trainee Clinical Research Network Study
Ophthalmology and Therapy (2017)