Abstract
Purpose
To compare the effect, failure rate and the risks of corneal cross-linking (CXL) in keratoconus patients aged ≥35 years to patients <35 years.
Methods
In 141 eyes of 116 keratoconus patients we compared the changes in best phoropter-corrected visual acuity (BCVA) and maximum keratometry values (Kmax) before and 12 months after CLX in patients aged ≥35 years (n=34, 38 eyes) to the cohort of patients below 35 years of age.
Results
Overall, CXL significantly improved BCVA from 0.487 logMAR (95% confidence interval (CI) 0.426–0.548) by −0.197 logMAR (95% CI −0.243 to −0.150; P<0.001) and reduced Kmax from 48.96 diopter (Dpt) by −1.33 Dpt (95% CI −1.85 to −0.81: P<0.001). Age ≥35 years had no effect on the changes of BCVA (−0.02 (95% CI −0.13 to 0.09); P=0.757) or Kmax (0.58 (95%CI −0.51 to 1.68); P=0.294) as compared with younger patients. In 54 patients (55 eyes, 38.5%) aged <35 years and in 18 patients (18 eyes, 47.4%) aged ≥35 years, BCVA increased by ≥2 Snellen lines. Failure (increase in Kmax ≥1 Dpt) was observed in 17 eyes (16.5%) of patients aged <35 years and in 3 eyes (7.9%) of patients aged ≥35 years during the 12-month follow-up period. Adverse outcomes (loss of ≥2 Snellen lines) occurred in 4 (3.9%) eyes of patients aged <35 years and 1 (2.6%) eye of a patient aged ≥35 years.
Conclusion
Effects and adverse events of CXL treatment do not seem to differ between subjects younger or older than 35 years.
Similar content being viewed by others
Introduction
Keratoconus (KC) is a corneal ectasia that typically progresses in young adults, but the rate of progression slows with increasing age.1 Corneal cross-linking (CXL) using riboflavin and ultraviolet-A light has been shown to effectively increase corneal stiffness and even to reduce ectasia.2, 3, 4 These findings originate from studies done mainly in young adults and led to the widespread use of CXL to stabilize KC in this group of patients, who have a 20% risk of progressing to a stage that requires therapeutic keratoplasty.
Thus far, little attention has been paid to the effect of CXL in KC patients older than 35 years of age. During ageing, a natural cross-linking pathway occurs, which involves a nonenzymatic reaction.5 The physiological cross-linking of the cornea may explain the slowdown of KC progression in older patients. Despite the reduced rate of progression of KC with increasing age, KC can progress in older patients, resulting in reduced visual acuity and quality of life.1, 6, 7, 8 Whether iatrogenic CXL in older adults with naturally cross-linked corneas has the same positive effect as in younger adults is unknown. A recent study on the safety of CXL found that age ≥35 years was a major risk factor for adverse events after CXL.9 Hence, it is arguable whether CXL should be offered to KC patients aged ≥35 years.
The aim of this study was to investigate the effect of CXL in KC patients aged ≥35 years. In particular, we analyzed the effect of CXL on visual acuity, keratometric conus regression, and adverse events in patients aged ≥35 years as compared with younger ones over a 12-month follow-up period.
Materials and methods
Study design
This retrospective, single-centre study enrolled patients with mild-to-moderate KC (Kmax <65.00 diopter) and a corneal thickness of ≥400 μm at the time of surgery. Patients who did not fulfill these two criteria were excluded from this analysis. Indication for treatment was based on progression of KC. Progression was verified by repeated corneal topography over at least 6 months, and defined as an increase in the central maximum keratometry value (Kmax) of ≥1.0 diopter (Dpt). Progression was also accepted if patients needed a new contact lens fitting because of a steepening of the cone more than once in the last 2 years, in cases of diurnal fluctuation of vision, or if patients developed contact lens intolerance (three patients). Eligible patients were aged between 16 and 70 years, and were not pregnant or breastfeeding at the time of the treatment. Exclusion criteria were ocular pathology other than keratoconus and previous refractive or other corneal surgery.
Examination
Best phoropter-corrected visual acuity (BCVA) was assessed at the initial consultation and at follow-up visits at 1, 3, and 12 months postoperatively using Snellen charts. Kmax was also measured at the initial consultation and the 1-, 3-, and 12-moth postoperative follow-ups by corneal topography (Precisio iVIS Technologies, Taranto, Italy).
CXL failure was defined as the percentage of eyes with an increase in Kmax of >1.0 Dpt 12 months after the intervention compared with the preoperative values.
The adverse event rate was defined as the percentage of eyes with a loss of ≥2 Snellen lines of BCVA at the 12-month follow-up compared with preoperative values.
Surgical technique
After topical anesthesia (proxymetacaine hydrochloride 5 mg/ml, Alcaine, Alcon Pharmaceuticals Ltd, Huenenberg, Switzerland) and insertion of a lid speculum, the epithelium was removed after a 10-s application of 94% ethanol (Laboratorium Dr Bichsel AG, Interlaken, Switzerland) in a 9.0-mm diameter area. The limbus was masked with instrument wipes (Visiwipe, Beaver-Visitec International, Waltham, MA, USA) to protect the stem cells from irradiation. Then 0.1% riboflavin, 20% dextran drops (Medio-cross, Peschke GmbH, Waldshut, Germany) were instilled every 3 min for 30 min. Stromal thickness during CXL was monitored with ultrasonic pachymetry (Corneo-Gage, Sonogage, Cleveland, OH, USA). In the event that pachymetry readings dropped below 400 μm, a hypotonic riboflavin solution (Vitamin B2 Streuli, Streuli Pharma, Uznach, Switzerland) was used to increase stromal thickness.10 Penetration of riboflavin into the aqueous humor was verified by slit lamp examination (blue light) before irradiating the eye for 30 min with ultraviolet-A light (370 nm) with an irradiance of 3 mW/cm2 (UV-X System; Peschke Meditrade GmbH, Huenenberg, Switzerland). During irradiation, 0.1% riboflavin drops were instilled every 5 min and further anesthetic drops if needed by the patient. After the procedure, a bandage contact lens (Air Optix Night&Day, Ciba Vision AG, Embrach, Switzerland) was applied until re-epithelialisation was complete on postoperative day 4. Topical antibiotic (ofloxacin 3 mg/ml, Floxal UD, Bausch&Lomb, Zug, Switzerland) 4 times daily was given for 5 days, the use of lubricating eye drops (Lacrycon SDU, Thea Pharma SA, Schaffhausen, Switzerland) was at the patient’s discretion. After epithelial healing, patients used topical fluorometholone (FMLLiquifilm, Allergan AG, Freienbach, Switzerland) 5 times daily for 4 weeks.
Statistical analysis
Interval scaled variates were summarized with means and SD or medians and interquartile ranges, where appropriate. Dichotomous variates were described as ratios and percentages. For statistical reasons, we expressed visual acuity with the logMAR (logarithm of the minimum angle of resolution=minus natural logarithm of BCVA). For both outcome parameters logMAR and Kmax, we first calculated the difference (logMAR after CLX−logMAR before CLX) and (Kmax before CLX−Kmax after CLX). To explore the strength of the association between logMAR or Kmax changes and patients’ age ≥35 years, we fitted two multivariate mixed linear models using subject as a random factor. These models adjusted for the fact that measurements were independent between subjects but dependent within subjects if a subject provided data on both eyes. This approach thus allowed analyzing data of all available eyes validly. To each model we entered an indicator variate for age ≥35 years. To adjust for potential confounders identified in a previous study,9 we also added indicator variates for preoperative BCVA better than 20/25 and preoperative maximum K readings >58.00 Dpt. All analyses were performed using the Stata 11.1 statistics software package (StataCorp LP, College Station, TX, USA).
Results
Patients’ characteristics
During the study period between January 2007 and January 2010, 166 eyes of 141 patients received CXL treatment for KC at our institution. During the 12-month follow-up period, 25 eyes of 25 patients were lost to follow-up. Hence, the final analysis included 141 cross-linked eyes of 116 KC patients. Detailed patient characteristics are given in Table 1.
Overall effects of CXL
For the overall population, CXL improved BCVA significantly from 0.487 logMAR (95% CI 0.426–0.548) by −0.197 logMAR (95% CI −0.243 to −0.150; P<0.001). The maximum K values regressed by −1.33 (95% CI −1.85 to −0.81: P<0.001) during the 12-month follow-up period.
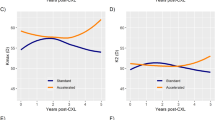
Effect of age on CXL
In KC patients undergoing CXL, age ≥35 years had no effect on the changes of BCVA (−0.02 (95% CI −0.13 to 0.09); P=0.757) or Kmax (0.58 (95%CI −0.51 to 1.68); P=0.294) as compared with patients <35 years of age even when correcting for potential confounders such as BCVA better than 20/25 and preoperative maximum K readings >58.00 Dpt. In 54 patients (55 eyes, 38.5%) aged <35 years, and in 18 patients (18 eyes, 47.4%) aged ≥35 years, BCVA increased by ≥2 Snellen lines. Also, for Kmax we found 12 months after CXL a nonsignificant interaction of −0.515 (95% CI −1.610 to 0.580: P=0.354) indicating a slightly larger reduction of Kmax in patients aged ≥35 years as compared with patients <35 years (Figure 1).
Treatment failures and rate of adverse events
Treatment failures (defined as increase in Kmax ≥1 Dpt 12 months after CXL) were observed in 17 eyes (16.5%) in patients aged <35 years, and in 3 eyes (7.9%) of patients aged ≥35 years (Fisher’s exact test P=0.278).
Adverse events of CXL (loss of ≥2 Snellen lines) were seen in 4 eyes (3.9%) of patients aged <35 years and 1 eye (2.6%) of a patient aged ≥35 years.
The overall findings did not change when excluding data of the second eye in patients providing data on both eyes.
Conclusion
In this 12-month CXL follow-up study, improvement of BCVA and Kmax and the number of adverse events did not seem to differ between subjects younger or older than 35 years.
Since the introduction of CXL over 10 years ago, several studies4, 11 proved the benefit of stopping KC progression with CXL. The effect of CXL found in our patients with regard to improved visual acuity and reduced keratometry is very similar to all other studies investigating the effect of CXL with epithelial removal in KC patients.2, 3, 4, 11, 12
An important objective of this study was to assess the risk of adverse events after CXL. Despite the overall enthusiasm for offering CXL to KC patients, adverse events have been incompletely assessed in the literature.13, 14 Koller et al9 reported an overall adverse event rate of 2.9% in 105 eyes, and identified age ≥35 years as the most important independent risk factor for adverse events after CXL. However, this findings needs to be interpreted cautiously, because, given the figures provided in the paper, the estimated number of eyes in patients aged >35 years enrolled in the Koller study was only 15 with 2 failures, corresponding to a mean probability of 0.13 and a binomial exact 95% CI of 0.02–0.40.
Our study in 141 eyes showed an adverse event rate of 3.9% in 82 patients (103 eyes) aged <35 years vs 2.6% in 34 patients (38 eyes) aged ≥35 years. All adverse events were due to stromal haze formation, which induced a loss of BCVA, except for one case in a young patient where a contact lens-induced epithelial problem was the reason for vision deterioration at the 12-month follow-up. Taking into account that stromal haze formation is a common finding after CXL15 and, because none of the patients experienced a severe complication, we still regard CXL as a relatively safe procedure in all age groups.
A limitation of our study is that the failure rate (7.9–16.5%), defined as increase of Kmax ≥1 Dpt after CXL, was somewhat higher than the failure rate reported in the literature (0–7.6%).9, 11, 12, 16 Arguably, the rate depends on the inclusion criteria, and further investigations are needed to define factors predicting the risk of failure. Including patients with high Kmax values could be one reason for the higher rate of failures. In our sample, however, this might not be relevant, because only seven patients with Kmax >58 Dpt were included and only one of them experienced a failure. Regarding the rate of adverse events, we did not objectively assess the extent of haze, which was the main cause for the persistent loss of vision after CXL. However, all clinical patient assessments were performed by the same experienced clinician, thereby limiting the extent of variability in judgment.
A potential problem of this retrospective analysis is the 25 (15.1%) patients lost to follow-up not attending the 12-month follow-up visit used to analyze the outcome data. We tried to contact the 25 lost patients by phone. We were able to reach 19 patients (14 aged <35 years and 5 aged ≥35 years). All 19 patients stated that they had missed their 1-year follow-up visit because they were satisfied with the outcome after CXL. Eighteen patients said that their vision had subjectively stayed unchanged or improved as compared with the time before CXL. One patient (aged 16 years) reported a slightly reduced vision, which did not bother him enough to attend the follow-up visit. We were unable to contact 6 patients (4 aged <35 years and 2 aged>35 years). Overall, patients’ feedback makes us confident that the substantial loss to follow-up has not biased our results substantially.
Because of the low number of adverse events, further prospective studies or a meta-analysis with larger patient numbers will be required to analyze the safety and complication rate of CXL. Using the approach of Blackwelder,17 and assuming that a difference in adverse effects between the two age groups of 5% or less would mean equivalence, the required sample size would be 283 patients per group with a power of 80% and an alpha level of 5%.
By assessing stromal haze formation after CXL and comparing stromal haze with BCVA, the complication rate might be reduced even more. Additional data may also help identify possible risk factors for failure of the intervention.
In conclusion, CXL improves visual acuity and reduces maximal keratometry values in KC patients aged ≥35 years as effectively as in younger patients. In addition, age did not seem to be a risk factor for adverse events, neither were preoperative BCVA better than 20/25 Snellen and a preoperative maximum K reading >58.00 Dpt. Therefore, CXL should be considered a valuable treatment option in patients aged ≥35 years, especially if they experience increasing CL intolerability or reduced visual acuity.

References
Wagner H, Barr JT, Zadnik K . Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: methods and findings to date. Cont Lens Anterior Eye 2007; 30 (4): 223–232.
Coskunseven E, Jankov MR 2nd, Hafezi F . Contralateral eye study of corneal collagen cross-linking with riboflavin and UVA irradiation in patients with keratoconus. J Refract Surg 2009; 25 (4): 371–376.
Hersh PS, Greenstein SA, Fry KL . Corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg 37 (1): 149–160.
Wittig-Silva C, Whiting M, Lamoureux E, Lindsay RG, Sullivan LJ, Snibson GR . A randomized controlled trial of corneal collagen cross-linking in progressive keratoconus: preliminary results. J Refract Surg 2008; 24 (7): S720–S725.
Malik NS, Moss SJ, Ahmed N, Furth AJ, Wall RS, Meek KM . Ageing of the human corneal stroma: structural and biochemical changes. Biochim Biophys Acta 1992; 1138 (3): 222–228.
Davis LJ, Schechtman KB, Wilson BS, Rosenstiel CE, Riley CH, Libassi DP et al. Longitudinal changes in visual acuity in keratoconus. Invest Ophthalmol Vis Sci 2006; 47 (2): 489–500.
Kymes SM, Walline JJ, Zadnik K, Sterling J, Gordon MO . Changes in the quality-of-life of people with keratoconus. Am J Ophthalmol 2008; 145 (4): 611–617.
McMahon TT, Szczotka-Flynn L, Barr JT, Anderson RJ, Slaughter ME, Lass JH et al. A new method for grading the severity of keratoconus: the Keratoconus Severity Score (KSS). Cornea 2006; 25 (7): 794–800.
Koller T, Mrochen M, Seiler T . Complication and failure rates after corneal crosslinking. J Cataract Refract Surg 2009; 35 (8): 1358–1362.
Hafezi F, Mrochen M, Iseli HP, Seiler T . Collagen crosslinking with ultraviolet-A and hypoosmolar riboflavin solution in thin corneas. J Cataract Refract Surg 2009; 35 (4): 621–624.
Wollensak G, Spoerl E, Seiler T . Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol 2003; 135 (5): 620–627.
Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE . Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg 2008; 34 (5): 796–801.
Herrmann CI, Hammer T, Duncker GI . [Hazeformation (corneal scarring) after cross-linking therapy in keratoconus]. Ophthalmologe 2008; 105 (5): 485–487.
Mazzotta C, Balestrazzi A, Baiocchi S, Traversi C, Caporossi A . Stromal haze after combined riboflavin-UVA corneal collagen cross-linking in keratoconus: in vivo confocal microscopic evaluation. Clin Experiment Ophthalmol 2007; 35 (6): 580–582.
Greenstein SA, Fry KL, Bhatt J, Hersh PS . Natural history of corneal haze after collagen crosslinking for keratoconus and corneal ectasia: Scheimpflug and biomicroscopic analysis. J Cataract Refract Surg 36 (12): 2105–2114.
Caporossi A, Baiocchi S, Mazzotta C, Traversi C, Caporossi T . Parasurgical therapy for keratoconus by riboflavin-ultraviolet type A rays induced cross-linking of corneal collagen: preliminary refractive results in an Italian study. J Cataract Refract Surg 2006; 32 (5): 837–845.
Blackwelder WC . ‘Proving the null hypothesis’ in clinical trials. Control Clin Trials 1982; 3 (4): 345–353.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Presented at the 114th Annual Meeting of American Academy of Ophthalmology (AAO), Chicago, IL, USA, 16–19 October 2010.
Rights and permissions
About this article
Cite this article
Baenninger, P., Bachmann, L., Wienecke, L. et al. Effects and adverse events after CXL for keratoconus are independent of age: a 1-year follow-up study. Eye 28, 691–695 (2014). https://doi.org/10.1038/eye.2014.56
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2014.56
This article is cited by
-
Early evaluation of corneal collagen crosslinking in ex-vivo human corneas using two-photon imaging
Scientific Reports (2019)