Abstract
Purpose
To evaluate the relationship between visual acuity as measured by the Early Treatment Diabetic Retinopathy Study (ETDRS) chart and by the potential acuity meter (PAM) with retinal thickness and sensitivity measured by a combined microperimetry/optical coherence tomography system (OCT).
Methods
Forty-four patients with macular edema (ME) were included in a prospective observational study. Visual acuity (VA) was assessed using the ETDRS chart (with best correction) as well as by the PAM. Retinal thickness and sensitivity was measured by an automatic fundus perimetry/tomography system.
Results
Best-corrected VA using the ETDRS chart ranged from 20/20 to 20/400 (median: 20/50). VA measured by the PAM without correction ranged from 20/20 to 20/400 (median: 20/40). The mean retinal thickness was 369.57 μm (s.d.: 140.28 μm) on spectral domain-OCT and the mean retinal sensitivity was 8.12 decibels (dB) (s.d.: 5.78 dB). The mean LogMAR value using the ETDRS chart was 0.43, whereas it was 0.38 using the PAM (P-value: 0.009).
Conclusions
VA values measured by the PAM were statistically significantly better than those measured by the ETDRS chart in eyes with ME secondary to various retinal vascular and uveitic diseases. VA measured by the PAM may be a more sensitive predictor of macular function than that obtained by ETDRS testing in eyes with ME.
Similar content being viewed by others
Introduction
Visual acuity (VA) is usually considered the most common clinical standard for determination of damage due to macular edema (ME); however, VA may be inadequate to describe the functional impairment caused by ME.1, 2, 3, 4 The conventional measurement of best-corrected visual acuity (BCVA) with the Early Treatment Diabetic Retinopathy Study (ETDRS) chart might be affected by anterior or posterior segment opacities and morbidities, including vitreous haze in the context of uveitis, as well as by ME secondary to uveitis, age-related macular degeneration (AMD), or diabetic retinopathy (DR).
The ‘Prevue’, a second-generation prototype of the Guyton–Minkowski potential acuity meter (PAM) is an instrument for measuring retinal VA behind a cataract or through otherwise clouded ocular media.5 The narrow beam of light traversing the eye toward each image point in the Prevue is less scattered in the presence of media opacity or ME compared with the full-pupil beam of light used for the measurement of VA by the standard ETDRS chart.6, 7 Thus, there should be a more accurate measurement of photoreceptor layer VA with the Prevue in the setting of media opacity or ME.8, 9, 10, 11, 12, 13, 14
Microperimetry has made it possible to correlate retinal function with the precise location of disease processes in the macula, thus providing an accurate evaluation of retinal function in relation to the retinal architecture and localized alterations of the retina caused by disease.1, 2, 15
The relationship between VA measured with the ETDRS chart and retinal architecture as well as function has been evaluated in different studies. A decrease in ETDRS-measured VA in eyes with diabetic ME (DME) and in those with uveitic ME (UME) has been reported.16, 17 The relationship between BCVA measured with the ETDRS chart and retinal sensitivity has been also reported. In previous studies, after adjustment for other factors such as retinal thickness, there has been no association between BCVA measured with the ETDRS chart and retinal sensitivity in eyes with DME and in those with UME.18, 19, 20 Roesel et al21 detected a significant negative correlation between retinal sensitivity and the logarithm of the minimum angle of resolution (LogMAR) values in eyes with UME.
To the best of our knowledge, there is no report on the relationship between VA measured by the ETDRS chart or the PAM/Prevue and retinal architecture and function. In the current study, we tested the hypothesis that whether a measurement of VA by the Prevue is a more accurate indicator of retinal function than that by the ETDRS chart in eyes with ME . To test this hypothesis, we conducted a longitudinal comparison of VA measured with the two systems in eyes with MA and assessed their relationship with retinal thickness as well as retinal sensitivity measured with an automatic fundus perimetry/tomography system.
Materials and methods
Forty-four patients (90 eye measurements) were enrolled in the index study. Inclusion criteria included the ability to provide written informed consent and comply with study assessments for the full duration of the study (∼1 h), age >18 years, and the presence of intermediate, posterior, or pan-uveitis or those with ME secondary to DR, AMD, vein occlusion, or uveitis. In addition, the study eyes had to achieve an ETDRS letter score, after a protocol refraction, of 18 or more letters (≥20/400 Snellen equivalent), had to have vitreous haze of ≤2+ as measured by standard National Eye Institute – Nussenblatt scales,22 and had to have ME detected by optical coherence tomography (OCT) defined by central subfield retinal thickness of ≥310 μm on spectral domain (SD)-OCT.
Patients with significant refractive errors such as high irregular astigmatism, which may affect the accurate measurement of VA with both the methods, patients with worse than 2+ nuclear sclerotic cataracts, and patients with corneal disease were excluded. The diagnosis of the ME from retinal diseases was made by using contact lens biomicroscopy, and confirmed with OCT and fluorescein angiography. The study and data accumulation were carried out with approval from the Johns Hopkins Institutional Review Board. The study was conducted in accordance with HIPAA regulations.
The best refractive correction was obtained using the ETDRS protocol by an experienced refractionist, and the BCVA was then assessed using an ETDRS chart. VA with the Prevue was obtained without correction, through the dilated pupil. The PAM is an FDA-approved device for the measurement of VA in a clinic setting. The device mounts onto a standard slit-lamp biomicroscope via a single pin that slips into the central pivot column of the instrument. The Prevue is a prototype of a second-generation PAM, not commercially available. It is basically a multiple-pinhole PAM that stands on a table. The patient leans forward and looks down ∼14° from the horizontal, along the internal optical path of the device, to see the image of a standard Snellen VA chart carried by multiple light beams far enough apart from one another that only one beam will fit within the pupil at any one time. The light beams narrow to their smallest diameter of about 0.15 mm, one-fourth the diameter of an ordinary straight pin. The device has a field of view of 6°. The patient reads the Snellen chart within the instrument, with line sizes from 20/400 to 20/20.
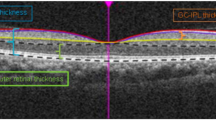
OCT imaging was performed using the Spectral OCT/SLO (Optos, Inc., Dunfermline, UK). Retinal thickness is defined as the distance between the inner limiting membrane and the hyporeflective line above the retinal pigment epithelium, and this distance is measured automatically by the OPKO software algorithm.23
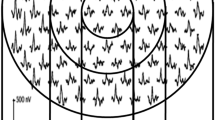
A modified microperimetry circular test pattern was applied to the eyes enrolled in the study. The following setting similar to the POLAR 3 test pattern was used in this study: Goldman-III stimulus size, 200 ms stimulus duration, and a 1000-ms interval between stimuli.24
Fundus localization based on retinal vessel alignment was automatically tracked by the Spectral OCT/SLO system. For each locus of the sensitivity measurement, retinal thickness values were obtained. The paired data of microperimetry threshold and corresponding retinal thickness measurements were used to evaluate the relationship between retinal thickness and VA adjusted for different retinal sensitivity values.23
The fixation pattern was evaluated with regard to fixation stability and fixation location. Fixation stability was classified using three categories: stable, relatively unstable, or unstable.25 If >75% of the fixation points were located within a 2° diameter circle, regardless of their position in relation to the foveal center, the fixation was classified as stable. If <75% of the fixation points were located within a 2° diameter circle, but >75% of the fixation points were located within a 4° diameter circle, fixation was classified as relatively unstable. If <75% of the fixation points were located within a 4° diameter circle, fixation was classified as unstable. Fixation location was designated to be in one of the three categories: central, pericentral, and eccentric.25 If 50% of the fixation points were within 0.5 mm of the foveal center, fixation was classified as central (0.5 mm subtends a visual angle of about 2.3°). If 25–50% of the fixation points were within 0.5 mm of the foveal center, fixation was classified as pericentral. If <25% of the fixation points were within 0.5 mm of the foveal center, fixation was classified as eccentric.
Statistical analysis
The details of statistical analysis are discussed in previous papers of this series.18, 20 To conduct the longitudinal comparison of VA measured by the ETDRS chart and by the Prevue, we defined a new variable, the so-called VA difference. VA difference was defined as the difference between the LogMAR value for BCVA measured by the ETDRS chart minus that for the VA measured by the Prevue without correction. Exploratory data analyses included graphical displays and statistical summaries that were performed. The potential confounding variables included retinal sensitivity, underlying disease, fixation stability, fixation location, age, and gender. Age was treated as a categorical variable with levels for age <42 years, ≥42 years and <55 years, ≥55 years and <75 years, and ≥75 years. The cut-points were selected based on exploratory data analysis.
Linear mixed models were used to estimate the VA difference as a function of retinal thickness, adjusting for the potential confounding variables. The relationship between the mean retinal thickness and VA difference was estimated separately for two separate groups of retinal thickness, those values of <590 μm, and those of ≥590 μm for both the measurement methods; the cut-point of 590 μm was selected on the basis of exploratory data analysis.
There were several potential sources of correlation within the data: measurements correlated within subjects, visits, eyes, and the circular test pattern (central and/or outer ring around the foveal center). Exploratory analyses of the potential sources of correlation were performed by fitting the mean model described above, assuming the data were independent, and examining the residuals of that model for the various sources of correlation. The linear mixed model for VA difference included random intercepts for subject and eye.26 This method of analysis has enabled us to adjust for the dependence of the data obtained from both the eyes of the same subjects and repeated measurements of the same subjects. The statistical analyses were performed using STATA version 10.1 (Statacorp, College Station, TX, USA). Statistical significance was reported if P<0.05.
Results
Ninety eye measurements of 44 eligible patients were included in this study. Among the 44 patients, 26 patients contributed both eyes (52 eyes/52 measurements) and 18 patients contributed one eye (18 eyes/18 measurements) at the initial visit. Twelve patients had follow-up visits and repeated measurements; 4 of them had repeated measurement on one eye (4 more measurements) and 8 had repeated measurement on both eyes, which added 16 more measurements to the total number of 90 measurements.
Thirty patients (68.2%) had DME, 10 (22.7%) UME, 1 (2.70%) AMD, and 3 (6.8%) had vein occlusion at the time of microperimetry measurement. The age of the patients ranged from 22.7 to 87.2 years (median: 62.3 years) and 59.0% were women (n=26).
Fifty-three eyes had completed data on fixation stability and 49 on fixation location. Thirty-one eyes (58.5%) had stable fixation, 19 eyes (35.8%) had relatively unstable fixation, and 3 eyes (5.7%) had unstable fixation. Thirty-Four eyes (69.4%) had central fixation, eight eyes (16.3%) had pericentral fixation, and seven eyes (14.3%) had eccentric fixation. The mean retinal thickness was 369.57 μm (s.d.: 140.28 μm) on SD-OCT and the mean retinal sensitivity was 8.12 dB (s.d.: 5.78 dB).
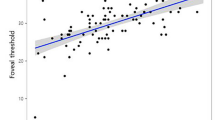
BCVA using the ETDRS chart ranged from 20/20 to 20/400 (median: 20/50). VA measured by the Prevue ranged from 20/20 to 20/400 (median: 20/40). Differences between the visual acuities measured with the two systems were evaluated using LogMAR values. The mean LogMAR value using the ETDRS chart was 0.43 (95% confidence interval (CI): 0.37, 0.49), whereas it was 0.38 (95% CI: 0.31, 0.45) using the Prevue (P-value: 0.009). The LogMAR values using the ETDRS chart as well as the Prevue were highly correlated (r=0.83) (Figure 1).
We reported the variable VA difference among different groups of retinal thickness measurements. The ranges were defined based on the available normal data as well as the s.d. of the mean. Figure 2 displays the relationship between the VA difference as a function of retinal thickness, estimated by a lowess smooth function (thick gray line). As it is seen in the figure, VA difference stayed above 0 for the entire range of retinal thickness, which presents a slightly higher LogMAR value (worse VA) measured by the ETDRS chart than with the Prevue. The change in VA difference is negligible for different values of retinal thickness. At a thickness of 200 μm, the VA difference is roughly 0.1, it decreases linearly to about 0 at a thickness of 590 μm, and then increases to about 0.1 at a thickness value of 800 μm. Figure 2 also presents the fitted model for a possible relationship between VA difference variable and retinal thickness (represented by the thick black line) as well as the 95% CI (dashed lines). Table 1 displays the estimated relationships between the VA difference variable and retinal thickness as well as the potential confounders based on the linear mixed-effects model. After removing the effects of retinal thickness and the potential confounders, there was a slight residual variation in VA, ∼88% of which could be attributed to differences between two eyes of each patient and 12% could be attributed to differences across patients. After accounting for the potential confounding variables, the VA difference decreased by an average 0.00001 LogMAR (95% CI: −0.00002, −1.68e-06) per 1 μm increase in the retinal thickness for the values of <590 μm. The VA difference increased by an average of 0.00004 LogMAR (95% CI: −0.00002, 0.00003) per 1 μm increase in the retinal thickness for values of ≥590 μm.
The relationship between the visual acuity difference and retinal thickness in patients with macular edema, estimated by a Lowess Smooth Function (thick gray line) as well as the fitted model for the relationship between retinal thickness and visual acuity (thick black line) and the 95% confidence interval (dashed lines). Visual acuity (VA) difference represents the difference between LogMAR values for best-corrected VA (BCVA) with the ETDRS chart and VA with the Prevue without correction.
Discussion
The BCVA measured by the ETDRS chart, even with its limitation in representing all different aspects of retinal function, has been the most commonly reported parameter to quantify the functional impact of ME.1, 2, 3, 4
There is evidence supporting the PAM as a useful instrument to evaluate retinal function in eyes with media opacities.8, 9, 10, 11 Barrett et al8 showed that the PAM successfully distinguished patients with retinal/neural disease from those without such disease in eyes with cataract. Datiles et al9 have recommended the PAM in high myopes with moderate cataracts and poor VA as a very reliable and helpful instrument in determining how much of the vision loss was due to the cataracts. Klein et al10 used the PAM to assess retinal function before neodymium-YAG laser posterior capsulotomy. They reported a good predictive value with the PAM, reducing the chances of the capsulotomy being a failure by fourfold. Steinert et al11 evaluated patients who underwent penetrating keratoplasty preoperatively for potential retinal acuity utilizing the PAM. They reported the PAM to be a useful qualitative predictor of retinal function when used in the presence of mild to moderate corneal edema. The PAM also has been shown to be a safe and quick method to predict VA after scleral buckling of macula-off retinal detachments, repair of macular holes, and after photodynamic therapy for subfoveal choroidal neovascularization due to AMD.12, 13, 14
Recently, microperimetry has added in-depth quantification of the function of the macula and has provided detailed information about the degree and pattern of retinal alteration in the course of edema.1, 2, 15, 18, 19 The integrated automated fundus perimetry/tomography and SD-OCT module of the Spectral OCT/SLO system has allowed an in-depth and more accurate evaluation of both retinal anatomy and corresponding function, with novel findings about their relationship as well as with associated factors. In our current study, we have measured the retinal thickness and sensitivity using the Spectral OCT/SLO device, which is an automatic, simultaneous fundus perimetry/tomography system, with a microperimetric circular pattern to cover the central 1 mm of the fixation center. We tested the difference in the measurement of BCVA using the ETDRS chart and VA using the Prevue for different retinal thickness and sensitivity values. We hypothesized that when retinal function stays stable, keeping sensitivity values constant in the statistical model, in eyes with ME the Prevue would be a better predictor of best potential VA and retinal function. To test this hypothesis, we defined a new variable, the so-called VA difference, which was calculated as BCVA measured with the ETDRS chart-VA measured with Prevue without correction. If the hypothesis is correct, for every 1 μm increase in retinal thickness there should be an increase in the VA difference variable while keeping other variables including retinal sensitivity constant. We tested this hypothesis with a linear mixed model for VA difference including random intercepts. The model took into account the variation in measurements across participants: two eyes of each patient, repeated visits/measurement of each eye, and several measurement points of each eye. As is shown in Figure 2, the VA difference variable stayed mainly positive for the majority of retinal thickness values. The lowess smooth function (thick gray line), which represents the mean of data stayed above 0 for the entire range of retinal thickness values. This means that the BCVA measured by the ETDRS chart was worse than the VA measured by the Prevue without correction (a positive difference indicates a higher LogMAR value with the ETDRS chart, which represents worse VA). The model also showed very negligible decrease in VA difference with each 1 μm increase in retinal thickness for values below 590 μm and a nonsignificant negligible increase in VA difference for those values above 590 μm. As it is shown in the model in Table 1, the LogMAR value has a 0.00001-unit decrease for each 1 μm increase in the retinal thickness for values below 590 μm. The P-value shows statistically significant difference. It means that, with 1-unit increase in retinal thickness for values below 590 μm, the difference between VA measured with the two methods becomes smaller and the two methods measure almost the same value. It is the opposite for retinal thickness values above 590 μm, where the difference in VA measured with the two methods increases with increase in retinal thickness values, and the ETDRS chart shows a worse performance compared with the PAM for retinal thickness above 590 μm.
Our findings suggest a slightly better VA measurement with the Prevue without correction compared with the BCVA measurement with the ETDRS chart. The difference did not change much with different retinal thickness values while other variables stayed constant. The impact of edema on VA measured with the ETDRS chart has been reported previously.16, 17 Sim et al16 reported a decrease in VA measured with the ETDRS chart in eyes with DME, whereas Taylor et al17 investigated the association between ME and impaired VA measured with the ETDRS chart in those with UME. To the best of our knowledge, our report is the first to evaluate the relationship between retinal thickness and VA as measured by the PAM/Prevue.
The model also revealed a negligible decrease in VA difference with a 1-dB increase in retinal sensitivity, but the finding was not statistically significant (Table 1). As shown in the model in Table 1, the LogMAR value has a 0.00007-unit decrease for each 1-dB increase in the retinal sensitivity. The P-value does not show statistically significant difference. It means that with 1-unit increase in retinal sensitivity the difference between VA measured with the two methods becomes smaller and the two methods measure almost the same value. Such an observation was confirmed by previous studies of eyes with DME and UME.18, 19, 20 Our findings were in contrast with some of the other reports.15, 21 Roesel et al21 showed a correlation between retinal sensitivity measured with the MP1 (Nidek Technologies, Padova, Italy) and BCVA measured by the ETDRS chart in eyes with UME. Carpineto et al15 presented a correlation between retinal sensitivity and BCVA measured by the ETDRS chart in DME eyes. Vujosevic et al1 detected a significant correlation between retinal sensitivity and BCVA measured with the ETDRS chart among diabetic patients with non-clinically significant ME. There was no association between retinal sensitivity and BCVA in eyes with DR and no ME, as well as in diabetic eyes with clinically significant ME. The main difference between the current study and their reports is the statistical modeling that we applied to our data set. The model took into consideration several potential sources of correlation within the data; measurements were correlated within subjects, visits, eyes, and the type of circular test pattern. The Pearson’s correlation coefficient as well as the generalized linear model applied in the other reports could be affected by these sources of correlation.
In the current study, fixation was reported to be stable and central in the majority of study eyes.2 However, our patients had better central fixation compared with those reported by Carpineto et al.15 Most of the patients in our study had DME as the underlying pathological mechanism, which was the same as in the study by Carpineto et al. However, other pathological mechanisms were included in the current study, which could potentially have a role in different patterns of retinal/choriocapillaris damage.21
There are several limitations that may have affected our results. The number of study patients is relatively small, and hence our results may not be generalizable. A larger sample size would allow us to further explore the relationship between retinal sensitivity and VA, and would also allow analyses of whether or not potential confounding factors such as prior laser photocoagulation, the duration of ME, and age have a role in retinal sensitivity. Other unadjusted factors in the model, such as the number of recurrent episodes of ME, the duration of underlying retinopathy, the number of flare-ups of uveitis in those with UME, fluctuation of blood glucose among DME patients, and previous medical intervention, as well as other ophthalmic conditions such as refractive errors, might also have a role in the relationship between retinal sensitivity and VA. The Prevue was used with dilated pupils without refractive correction in place for the majority of the patients. If refractive correction had been worn, the Prevue visual acuities would likely have been still better. During the course of the study, different examiners performed VA measurement using the Prevue. Although the device is easy to use in a clinic setting, there might be some inter-examiner variability in BCVA values measured with the Prevue. In addition, another limitation of our study is its inability to account for the level of difficulty between the ETDRS chart and the chart in PAM. In the ETDRS acuity system, it is the LogMAR chart with the same number of letters in each row. Therefore, the difficulty of each row should be similar. On the other hand, the chart used in PAM is a Snellen chart, which does not have the same number of letters in each row, leading to possible different levels of difficulties in each row. Such differences may affect the accuracy of our results.
Our pilot study has provided novel information on the relationship among VA, retinal thickness, and retinal sensitivity, as well as other parameters related to underlying retinopathies in patients with ME. Although costly and time-consuming techniques would provide detailed information regarding the macular function in eyes with media opacity or ME, the PAM/Prevue is a safe, quick, and readily available method to evaluate retinal VA in these eyes. Additional studies on microperimetry and measurements of VA in patients with ME may eventually yield a reliable and reproducible method of quantifying macular function.

References
Vujosevic S, Midena E, Pilotto E, Radin PP, Chiesa L, Cavarzeran F . Diabetic macular edema: correlation between microperimetry and optical coherence tomography findings. Invest Ophthalmol Vis Sci 2006; 47: 3044–3051.
Vujosevic S, Pilotto E, Bottega E, Benetti E, Cavarzeran F, Midena E . Retinal fixation impairment in diabetic macular edema. Retina 2008; 28: 1443–1450.
Larsson J, Zhu M, Sutter F, Gillies MC . Relation between reduction of foveal thickness and visual acuity in diabetic macular edema treated with intravitreal triamcinolone. Am J Ophthalmol 2005; 139: 802–806.
Campochiaro PA . Reduction of diabetic macular edema by oral administration of the kinase inhibitor PKC412. Invest Ophthalmol Vis Sci 2004; 45: 922–931.
Minkowski JS, Palese M, Guyton DL . Potential acuity meter using a minute aerial pinhole aperture. Ophthalmology 1983; 90: 1360–1368.
Guyton DL . The Reliability of Retinal Visual Acuity Measurements Behind Cataracts, Introduction to Chapter 3, Vision, Refraction, and Contact Lenses. In: Ernest T (ed.) The Year Book of Ophthalmology. Year Book Medical Publishers: Chicago, IL, USA, 1984 pp 55–58.
Guyton DL . Preoperative Visual Acuity Evaluation. Int Ophthalmol Clin 1987; 27: 140–148.
Barrett BT, Davison PA, Eustace P . Clinical comparison of three techniques for evaluating visual function behind cataract. Eye (Lond) 1995; 9: 722–727.
Datiles MB, Edwards PA, Kaiser-Kupfer MI, McCain L, Podgor M . A comparative study between the PAM and the laser interferometer in cataracts. Graefes Arch Clin Exp Ophthalmol 1987; 225: 457–460.
Klein TB, Slomovic AR, Parrish RK 2nd, Knighton RW . Visual acuity prediction before neodymium-YAG laser posterior capsulotomy. Ophthalmology 1986; 93: 808–810.
Steinert RF, Minkowski JS, Boruchoff SA . Pre-keratoplasty potential acuity evaluation. laser interferometer and potential acuity meter. Ophthalmology 1984; 91: 1217–1221.
Smiddy WE, Thomley ML, Knighton RW, Feuer WJ . Use of the potential acuity meter and laser interferometer to predict visual acuity after macular hole surgery. Retina 1994; 14: 305–309.
Friberg TR, Eller AW . Prediction of visual recovery after scleral buckling of macula-off retinal detachments. Am J Ophthalmol 1992; 114: 715–722.
Smith BT, Vander JE, Regillo CD, Ho AC . Evaluation of the potential acuity meter in predicting visual acuity after photodynamic therapy for choroidal neovascularization. Ophthalmic Surg Lasers Imaging 2006; 37: 6–11.
Carpineto P, Ciancaglini M, Di Antonio L, Gavalas C, Mastropasqua L . Fundus microperimetry patterns of fixation in type 2 diabetic patients with diffuse macular edema. Retina 2007; 27: 21–29.
Sim DA, Keane PA, Zarranz-Ventura J . The effect of macular ischemia on visual acuity in diabetic retinopathy. Invest Ophthalmol Vis Sci 2013; 54: 2353–2360.
Taylor SR, Lightman SL, Sugar EA . The impact of macular edema on visual function in intermediate, posterior, and panuveitis. Ocul Immunol Inflamm 2012; 20: 171–181.
Hatef E, Colantuoni E, Wang J, Ibrahim M, Shulman M, Adhi F et al. The relationship between macular sensitivity and retinal thickness in eyes with diabetic macular edema. Am J Ophthalmol 2011; 152: 400–405.
Kube T, Schmidt S, Toonen F, Kirchhof B, Wolf S . Fixation stability and macular light sensitivity in patients with diabetic maculopathy: a microperimetric study with a scanning laser ophthalmoscope. Ophthalmologica 2005; 219: 16–20.
Sepah YJ, Hatef E, Colantuoni E, Wang J, Shulman M, Adhi FI et al. Macular sensitivity and fixation patterns in normal eyes and eyes with uveitis with and without macular edema. J Ophthalmic Inflamm Infect 2012; 2: 65–73.
Roesel M, Heimes B, Heinz C, Henschel A, Spital G, Heiligenhaus A . Comparison of retinal thickness and fundus-related microperimetry with visual acuity in uveitic macular oedema. Acta Ophthalmol 2011; 89: 533–537.
Nussenblatt RB, Peterson JS, Foster CS, Rao NA, See RF, Letko E et al. Initial evaluation of subcutaneous daclizumab treatments for noninfectious uveitis: a multicenter noncomparative interventional case series. Ophthalmology 2005; 112: 764–770.
Landa G, Rosen RB, Garcia PM, Seiple WH . Combined three-dimensional spectral OCT/SLO topography and microperimetry: steps toward achieving functional spectral OCT/SLO. Ophthalmic Res 2010; 43: 92–98.
Anastasakis A, McAnany JJ, Fishman GA, Seiple WH . Clinical value, normative retinal sensitivity values, and intrasession repeatability using a combined spectral domain optical coherence tomography/scanning laser ophthalmoscope microperimeter. Eye (Lond) 2011; 25: 245–251.
Fujii GY, De Juan E Jr, Humayun MS . Characteristics of visual loss by scanning laser ophthalmoscope microperimetry in eyes with subfoveal choroidal neovascularization secondary to age-related macular degeneration. Am J Ophthalmol 2003; 136: 1067–1068.
Liang KY, Diggle PJ, Zeger SL . Analysis of Longitudinal Data 2nd edn. Oxford University Press: Oxford, UK, 2002.
Acknowledgements
The scanning laser ophthalmoscope/spectral domain optical coherence tomography unit (SLO/OCTTM) is on loan from Optos, Inc., UK, to the Johns Hopkins Medical Institution for research and clinical care. The study was supported in part through a Physician Scientist Award from Research to Prevent Blindness (QDN), as well as by the Macular Research Fund and the Pediatric Ophthalmology and Strabismus Research Grant Award, Wilmer Eye Institute, the Johns Hopkins University School of Medicine (EH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
The study was presented in part at the Association for Research in Vision and Ophthalmology Annual Meeting, May 2012, Fort Lauderdale, Florida.
Disclaimer
Dr Guyton was one of the inventors of the PAM and the Prevue prototype but no longer has any financial interest in their sale or use. The sponsor or funding organization had no role in the design or conduct of this research.
Rights and permissions
About this article
Cite this article
Hatef, E., Hanout, M., Moradi, A. et al. Longitudinal comparison of visual acuity as measured by the ETDRS chart and by the potential acuity meter in eyes with macular edema, and its relationship with retinal thickness and sensitivity. Eye 28, 1239–1245 (2014). https://doi.org/10.1038/eye.2014.182
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2014.182
This article is cited by
-
Topical ketorolac as an adjunctive treatment with intravitreal bevacizumab in the management of diabetic macular edema: A double-masked placebo-controlled randomized clinical trial
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)
-
Visual rehabilitation via microperimetry in patients with geographic atrophy: a pilot study
International Journal of Retina and Vitreous (2017)