Abstract
Purpose
In a retrospective review, the functional effects of intra-vitreal ranibizumab monotherapy in patients with sub-foveal haemorrhage secondary to choroidal neovascularisation (CNV) in age-related macular degeneration (ARMD) are reported.
Patients and methods
Twelve eyes of 12 patients were treated with intra-vitreal ranibizumab (0.5 mg in 0.05 ml) in accordance with current practice. Follow-up was arranged at monthly intervals. Eleven patients completed 6 months and seven completed 12 months of follow-up. Duration of haemorrhage, lesion size, ETDRS letter score at baseline, and follow-up were analysed.
Results
The mean time for complete clearance of sub-retinal haemorrhage was 4.7 months (range 3–7 months). After 6 months, visual acuity was stable or improved in 7 of 11 eyes and the mean change in ETDRS letter score was +7.6 letters (P>0.05). After 12 months, visual acuity was stable or improved in five of seven eyes and the mean change in ETDRS letter score was +7.3 letters (P>0.05). For the seven cases with 12 months of follow-up, a mean of six injections were given. Two patients had a decrease in acuity of >15 ETDRS letters during follow-up. No other local or systemic side-effects were reported.
Conclusions
Intra-vitreal ranibizumab monotherapy may be an effective treatment for sub-foveal haemorrhage secondary to CNV in ARMD.
Similar content being viewed by others
Introduction
Sub-foveal haemorrhage secondary to age-related macular degeneration (ARMD) has earlier been shown to have a poor prognosis.1 In an effort to counter this, several treatment approaches have earlier been evaluated. The Submacular Surgery Trial reported that surgical removal of the blood and associated choroidal neovascularisation (CNV) did not favour stable or improved vision for predominantly haemorrhagic lesions.2 Since then, pneumatic displacement of sub-retinal haemorrhage with or without tissue plasminogen activator (TPA) and sub-retinal TPA with removal of sub-retinal haemorrhage have also been evaluated. These techniques have been shown to be effective in displacing sub-foveal haemorrhage and this is often associated with an improvement in visual acuity.3, 4, 5 However, the longer-term effects seem to be limited by progression of the underlying CNV. Although patients with sub-foveal haemorrhage greater than a disc area or >50% of total lesion area were not included in the ranibizumab phase 3 trials, other authors have shown a benefit from combination therapy or monotherapy using drugs directed against vascular endothelial growth factor.6, 7, 8, 9 The aim of this study was to report the efficacy and safety of ranibizumab monotherapy in the treatment of sub-foveal haemorrhage secondary to neovascular ARMD.
Materials and methods
In the retrospective study, the medical records of 12 patients with ARMD and thick sub-foveal haemorrhage secondary to CNV were reviewed. Patients were assessed and treated in one of two centres, namely St James's University Hospital, Leeds, or the Hull and East Yorkshire Eye Hospital. After a discussion of the treatment options, each patient gave written consent for a course of treatment with intra-vitreal injections of ranibizumab 0.5 mg alone. Treatment was given according to standard practice with an initial loading phase of three consecutive injections at monthly intervals, followed by a maintenance phase with further assessment every month with repeat injection as judged necessary by the treating ophthalmologist and using recognised criteria.10 To be eligible for this retrospective review, the foveal haemorrhage had to be sufficient to elevate the fovea on clinical examination and/or to block the background choroidal fluorescence on fluorescein angiography. Patients also needed to have completed a minimum follow-up of 3 months.
Fundus photography and/or fluorescein angiography was performed before the initiation of treatment. The area of sub-retinal haemorrhage and the total lesion area was determined from these images. Selected patients had additional imaging after the resolution of the sub-retinal haemorrhage. At each clinical visit for assessment±treatment, patients underwent ETDRS acuity, OCT examination, and slit lamp examination of the anterior and posterior segments. For this study, the following data was recorded for each patient: age, gender, eye affected, duration of haemorrhage before treatment in weeks, ETDRS acuity at baseline and after treatment, baseline area of sub-retinal haemorrhage, total lesion area, and time for complete resolution of sub-retinal haemorrhage. The change in ETDRS acuity and the number of injections was recorded for those patients completing 3, 6, 9, and 12 months of follow-up. The Wilcoxon-signed rank test was used to compare the initial and follow-up visual acuities.
Results
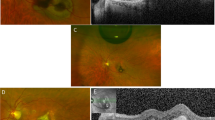
Twelve patients were treated in the two centres. The mean age was 80.4 years and four cases were male. The mean duration of sub-foveal haemorrhage, according to the patients’ symptoms, was 6 weeks (range 1–16 weeks) before the initiation of treatment. In half the cases, the haemorrhage was present for >4 weeks. The mean area of sub-retinal haemorrhage at baseline was 14.6 mm2 (range 0.5–45.4 mm2) and the mean total lesion area was 18.3 mm2 (range 0.5–47.3 mm2). In 9 of the 12 cases, the haemorrhage comprised >50% of the total lesion area. For the 11 cases in which the sub-retinal haemorrhage cleared during follow-up, the mean time for the haemorrhage to clear was 4.7 months (range 3–7 months) (see Figure 1). This data is summarised in Table 1.
Baseline colour fundus photograph of Case 4, with early and late phase fluorescein angiography at baseline and fundus photograph after 3 months of follow-up, with complete resolution of sub-retinal haemorrhage. Hyperfluorescence is seen around the blocked fluorescence in the late phase of the angiogram. Visual acuity improved by 15 letters at month 12.
The mean visual acuity at baseline was 46 ETDRS letters (median=45, range 19–73). A single patient missed one injection during the initial phase of treatment. For the 12 patients with at least 3 months of follow-up, the mean acuity change was −1.6 letters (range −44 to +12). Seven cases had a gain of at least one ETDRS letter compared with baseline and the remaining five cases had no change or a decrease in acuity. A single case had a decrease of >15 letters (see Table 2). No significant difference was seen between the cases with haemorrhage >50% of the total lesion area compared with <50%, or of duration ⩽4 weeks compared with >4 weeks, or with baseline acuity <45 letters compared with ⩾45 letters (see Table 1).
For the 11 patients with at least 6 months of follow-up, the mean acuity change was +7.6 ETDRS letters (range −11 to +25). Seven cases had a gain of at least one ETDRS letter and the remaining four cases had no change or a decrease in ETDRS letter score. For the seven patients with at least 12 months follow-up, the mean acuity change was +7.3 ETDRS letters (range −16 to +20). Five cases had a gain of at least one ETDRS letter compared with baseline and the remaining two cases had a decrease in acuity (see Tables 1 and 2).
A single patient missed one injection during the initial loading phase of treatment. During the maintenance phase, patients received a mean of 1.5 intra-vitreal injections (n=11, range 0–3) from months 3–5 and three injections (n=7, range=0–7) from months 3–11. For the seven cases with 12 months of follow-up, the mean number of injections from baseline up to and including the month 11 visit was six (range 3–11).
Two patients had a decrease of >15 ETDRS letters during the follow-up period. A single patient, with a baseline acuity of 45 ETDRS letters and the largest sub-retinal haemorrhage, had a vitreous haemorrhage between 2 and 3 months of follow-up and a decrease in ETDRS letter score of 44 ETDRS letters. The vitreous haemorrhage failed to clear spontaneously and a pars plana vitrectomy was performed for persistent vitreous haemorrhage between 5 and 6 months of follow-up. Post-operatively, the vitreous haemorrhage recurred and the acuity at final follow-up was hand movements only. Another patient had a decrease in acuity of 16 letters recorded at the 12-month examination. This decrease in acuity was noted at every examination from baseline. At the 12-month examination, sub-retinal fibrosis was noted in addition to persistent intra-retinal oedema on OCT imaging. No other significant ocular or systemic adverse events were reported for these or the other patients.
Discussion
In this retrospective study, we report the effect of ranibizumab monotherapy as a treatment for CNV secondary to ARMD, complicated by sub-foveal haemorrhage. Although the follow-up is not complete, the overall results in this series of 12 eyes are encouraging, with over half the eyes having some gain in ETDRS letter score at each of the quarterly visits completed after baseline. Only two eyes had moderate visual loss during the follow-up, because of vitreous haemorrhage requiring pars plana vitrectomy and sub-retinal fibrosis, respectively.
At the 3-month visit, 58% of the cases in this series had a modest gain in EDTRS letter score and 33% had a decrease. This is similar to that reported by Sacu et al6, using bevacizumab monotherapy, in which 6 of 10 cases had a gain and 4 had a decrease in acuity at 4 months. The mean area of haemorrhage and the time to resolution was also comparable with our series, although the mean duration of haemorrhage before treatment was <2 weeks. Although Sacu suggested a superior outcome at 4 months when bevacizumab therapy was combined with an initial injection of TPA and expansile gas, six cases in our series had their highest ETDRS letter score at a quarterly visit after the month 3 examination, suggesting that the maximal benefit of ranibizumab monotherapy may often be delayed beyond this point. Stifter et al reported improved acuity in 48% of 21 cases and reduced acuity in 43% after 4 months of bevacizumab monotherapy.7 At 12 months, the 25% had improved and 58% had reduced acuity. In that series, the area of haemorrhage at baseline was also similar and in 33% of cases, the haemorrhage was 30 days or more old. However, only 9 of the 21 cases had sub-foveal haemorrhage at baseline, although the paper reports no significant difference in outcome between eyes with sub-foveal or extra-foveal haemorrhage at baseline.
The duration of sub-foveal haemorrhage in this series varied from 1 to 16 weeks before treatment, according to patient history. The extent of sub-retinal haemorrhage was also variable, ranging from 0.5 to 45 mm2. In all cases, the haemorrhage extended under the foveal centre and was sufficient to elevate the fovea on clinical examination and/or block choroidal fluorescence on fluorescein angiography. Despite this, baseline acuity was between 19 and 73 ETDRS letters. Similar levels of acuity have been documented in other series.3, 6 It has been suggested that both the extent and the prolonged presence of sub-retinal blood under the fovea may have a poor visual prognosis, possibly because of iron toxicity, shearing forces, and blockage of oxygen distribution.11, 12 Similarly, baseline acuity may influence final acuity.12 None of these features appeared to influence outcome in this series.
After the initial, loading phase, the mean number of injections was 1.5 to month 5 and 3.3 to month 11. All seven patients completing the 12-month follow-up visit had fewer injections in the second 6 months than in the first 6 months. A single patient required no additional injections during the maintenance phase. The visual acuity trend during the maintenance phase reflected that seen in other trials with an identical approach to treatment.13
One significant complication in this series was a vitreous haemorrhage requiring vitrectomy in an eye with a large, predominantly haemorrhagic lesion. After this procedure, there was further haemorrhage into the vitreous cavity and no view of the retina was obtained. Vitreous haemorrhage was noted in 17% of cases in the control arm of the Submacular Surgery Trial.2 Retinal pigment epitheial rip and an increase in sub-macular haemorrhage have been documented with bevacizumab therapy.13, 14 These complications, which seem to be more common with larger lesions and with specific characteristics, need to be considered as potential complications of treatment.15
In this retrospective series, ranibizumab monotherapy was an effective treatment for sub-foveal haemorrhage secondary to CNV in ARMD. These results are comparable with those of studies in which therapy against vascular endothelial growth factor is combined with surgery to displace sub-foveal haemorrhage, suggesting that the displacement of sub-foveal haemorrhage may not be necessary.

References
Avery R, Fekrat S, Hawkins B, Bressler N . Natural history of subretinal subfoveal haemorrhage in age-related macular degeneration. Retina 1996; 16: 183–189.
Bressler NM, Bressler SB, Childs AL, Haller JA, Hawkins BS, Lewis H, et al., Submacular Surgery Trials (SST) Research Group. Surgery for hemorrhagic choroidal neovascular lesions of age-related macular degeneration: ophthalmic findings: SST report no. 13. Ophthalmology 2004; 111 (11): 1993–2006.
Olivier S, Chow DR, Packo KH, MacCumber MW, Awh CC . Subretinal recombinant tissue plasminogen activator injection and pneumatic displacement of thick submacular hemorrhage in age-related macular degeneration. Ophthalmology 2004; 111 (6): 1201–1208.
Gopalakrishan M, Giridhar A, Bhat S, Saikumar SJ, Elias A, N S . Pneumatic displacement of submacular hemorrhage: safety, efficacy, and patient selection. Retina 2007; 27 (3): 329–334.
Singh RP, Patel C, Sears JE . Management of subretinal macular haemorrhage by direct administration of tissue plasminogen activator. Br J Ophthalmol 2006; 90 (4): 429–431.
Sacu S, Stifter E, Vécsei-Marlovits PV, Michels S, Schütze C, Prünte C et al. Management of extensive subfoveal haemorrhage secondary to neovascular age-related macular degeneration. Eye 2009; 23 (6): 1404–1410.
Stifter E, Michels S, Prager F, Georgopoulos M, Polak K, Hirn C et al. Intravitreal bevacizumab therapy for neovascular age-related macular degeneration with large submacular hemorrhage. Am J Ophthalmol 2007; 144 (6): 886–892.
Meyer CH, Scholl HP, Eter N, Helb HM, Holz FG . Combined treatment of acute subretinal haemorrhages with intravitreal recombined tissue plasminogen activator, expansile gas and bevacizumab: a retrospective pilot study. Acta Ophthalmol 2008; 86 (5): 490–494.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al., MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355 (14): 1419–1431.
Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 2007; 143 (4): 566–583.
Hattenbach LO, Klais C, Koch FH, Gümbel HO . Intravitreous injection of tissue plasminogen activator and gas in the treatment of submacular hemorrhage under various conditions. Ophthalmology 2001; 108 (8): 1485–1492.
Schulze SD, Hesse L . Tissue plasminogen activator plus gas injection in patients with subretinal hemorrhage caused by age-related macular degeneration: predictive variables for visual outcome. Graefes Arch Clin Exp Ophthalmol 2002; 240 (9): 717–720.
Holz FG, Meyer C, Eter N . Safety and efficacy of ranibizumab treatment in patients with neovascular age-related macular degeneration: 12-month results of the SUSTAIN study. Invest Ophthalmol Vis Sci 2009; 50; E-Abstract 3095.
Chan CK, Meyer CH, Gross JG, Abraham P, Nuthi AS, Kokame GT et al. Retinal pigment epithelial tears after intravitreal bevacizumab injection for neovascular age-related macular degeneration. Retina 2007; 27 (5): 541–551.
Goverdhan SV, Lochhead J . Submacular haemorrhages after intravitreal bevacizumab for large occult choroidal neovascularisation in age-related macular degeneration. Br J Ophthalmol 2008; 92 (2): 210–212.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Part of this work was presented as a Poster presentation at the Annual Congress of the Royal College of Ophthalmologists, 2009
Rights and permissions
About this article
Cite this article
McKibbin, M., Papastefanou, V., Matthews, B. et al. Ranibizumab monotherapy for sub-foveal haemorrhage secondary to choroidal neovascularisation in age-related macular degeneration. Eye 24, 994–998 (2010). https://doi.org/10.1038/eye.2009.271
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2009.271
Keywords
This article is cited by
-
Incidence of submacular haemorrhage (SMH) in Scotland: a Scottish Ophthalmic Surveillance Unit (SOSU) study
Eye (2019)
-
Quantification of retinal changes after resolution of submacular hemorrhage secondary to polypoidal choroidal vasculopathy
Japanese Journal of Ophthalmology (2018)
-
Intravitreal anti-vascular endothelial growth factor monotherapy for large submacular hemorrhage secondary to neovascular age-related macular degeneration
Eye (2015)