Abstract
Objective
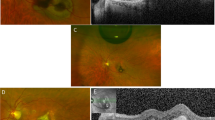
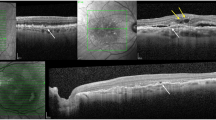
To compare efficacy and safety profile of subretinal aflibercept, ranibizumab, and bevacizumab in the context of pars plana vitrectomy, pneumatic displacement with subretinal air and subretinal tPA for subretinal macular haemorrhage (SMH) due to naïve neovascular age-related macular degeneration (nAMD).
Design
Retrospective interventional cohort study.
Participants
123 eyes of 123 patients treated with subretinal aflibercept (n = 41, 33%), ranibizumab (n = 41,33%), and bevacizumab (n = 41, 33%).
Methods
Review of electronic medical records for best corrected visual acuity (BCVA), central subfoveal thickness (CST), and intraocular pressure (IOP) at baseline and 24 months after treatment.
Main outcome measures
BCVA, CST, and number of intravitreal anti VEGF over 24 months.
Results
Mean age of patients was 80.5 ± 5.5 years, 43.9% were female. Mean time from symptom onset until surgery was 1.1 days (range 0–3 days). In all cases, the SMH did not reach the arcades. CST at baseline was 627 ± 140 µ, 739 ± 54 µ, and 793 ± 93 µ (p = 0.0001) for aflibercept, ranibizumab, or bevacizumab, respectively. Baseline BCVA (logMAR) was 0.65 ± 0.13, 0.69 ± 0.96, and 0.74 ± 0.81 (p = 0.0041) for aflibercept, ranibizumab, and bevacizumab, respectively. All three groups showed statistically significant improvement in BCVA and CST (for all groups: p < 0.001). There was no statistically significant difference at the final BCVA (p = 0.789). The mean number of anti VEGF given during follow-up period was 5.2 ± 0.81, 4.4 ± 0.63, and 5.5 ± 0.95 (p = 0.0001) for aflibercept, ranibizumab, and bevacizumab, respectively.
Conclusion
This study shows that aflibercept, ranibizumab, and bevacizumab in a subretinal manner in the context of PPV, pneumatic displacement with subretinal air and subretinal tPA for subretinal macular haemorrhage secondary to naïve nAMD work with the same efficacy and safety profile.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this paper or its supplementary material files. Further enquiries can be directed to the corresponding author.
References
Toth CA, Morse LS, Hjelmeland LM, Landers MB. Fibrin directs early retinal damage after experimental subretinal hemorrhage. Arch Ophthalmol (Chic, Ill 1960). 1991;109:723–9.
Scupola A, Coscas G, Soubrane G, Balestrazzi E. Natural history of macular subretinal hemorrhage in age-related macular degeneration. Ophthalmologica. 1999;213:97–102.
Glatt H, Machemer R. Experimental subretinal hemorrhage in rabbits. Am J Ophthalmol. 1982;94:762–73.
Haupert CL, McCuen BW 2nd, Jaffe GJ, Steuer ER, Cox TA, Toth CA, et al. Pars plana vitrectomy, subretinal injection of tissue plasminogen activator, and fluid-gas exchange for displacement of thick submacular hemorrhage in age-related macular degeneration. Am J Ophthalmol 2001;131:208–15.
Olivier S, Chow DR, Packo KH, MacCumber MW, Awh CC. Subretinal recombinant tissue plasminogen activator injection and pneumatic displacement of thick submacular hemorrhage in Age-Related macular degeneration. Ophthalmology 2004;111:1201–8.
Martel JN, Mahmoud TH. Subretinal pneumatic displacement of subretinal hemorrhage. JAMA Ophthalmol. 2013;131:1632–5.
Chang W, Garg SJ, Maturi R, Hsu J, Sivalingam A, Gupta SA, et al. Management of thick submacular hemorrhage with subretinal tissue plasminogen activator and pneumatic displacement for age-related macular degeneration. Am J Ophthalmol. 2014;157:1250–7.
Stopa M, Lincoff A, Lincoff H. Analysis of forces acting upon submacular hemorrhage in pneumatic displacement. Retina 2007;27:370–4.
Iglicki M, Khoury M, Melamud JI, Donato L, Barak A, Quispe DJ, et al. Naïve subretinal haemorrhage due to neovascular age-related macular degeneration. pneumatic displacement, subretinal air, and tissue plasminogen activator: subretinal vs intravitreal aflibercept-the native study. Eye (Lond). 2023;37:1659–64.
Klettner A, Grotelüschen S, Treumer F, Roider J, Hillenkamp J. Compatibility of recombinant tissue plasminogen activator (rtPA) and aflibercept or ranibizumab coapplied for neovascular age-related macular degeneration with submacular haemorrhage. Br J Ophthalmol. 2015;99:864–9.
Avci R, Kapran Z, Ozdek Ş, Teke MY, Oz O, Guven D, et al. Multicenter study of pars plana vitrectomy for optic disc pit maculopathy: MACPIT study. Eye 2017;31:1266–73.
The CATT Research Group, The CRG. Ranibizumab and bevacizumab for neovascular. N Engl J Med. 2011;364:1897–908.
Matsuoka M, Ogata N, Otsuji T, Nishimura T, Takahashi K, Matsumura M. Expression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol. 2004;88:809–15.
Jackson TL, Bunce C, Desai R, Hillenkamp J, Lee CN, Lois N, et al. Vitrectomy, subretinal Tissue plasminogen activator and Intravitreal Gas for submacular haemorrhage secondary to Exudative Age-Related macular degeneration (TIGER): study protocol for a phase 3, pan-European, two-group, non-commercial, active-control, ob. Trials 2022;23:99.
Lüke M, Januschowski K, Warga M, Beutel J, Leitritz M, Gelisken F, et al. The retinal tolerance to bevacizumab in co-application with a recombinant tissue plasminogen activator. Br J Ophthalmol. 2007;91:1077–82.
Singh RP, Patel C, Sears JE. Management of subretinal macular haemorrhage by direct administration of tissue plasminogen activator. Br J Ophthalmol. 2006;90:429–31.
de Silva SR, Bindra MS. Early treatment of acute submacular haemorrhage secondary to wet AMD using intravitreal tissue plasminogen activator, C3F8, and an anti-VEGF agent. Eye (Lond). 2016;30:952–7.
Acknowledgements
Anat Loewenstein and Dinah Zur, Tel Aviv Medical Center, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Author information
Authors and Affiliations
Contributions
MI, DJQ, MK. - Substantial contributions to the conception or design of the work; the acquisition, analysis, and interpretation of data for the work. - Drafting the work or revising it critically for important intellectual content. - Final approval of the version to be published. - Ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. JIM, LD, HPN - Substantial contributions to the conception or design of the work and interpretation of data for the work. - Drafting the work or revising it critically for important intellectual content. - Final approval of the version to be published. - Ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submit- ted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethics approval
As this was a retrospective study, participants' informed consent was not needed, in compliance with the Institutional review board (IRB) approval. This study protocol was reviewed and approved by the local IRB. The research adhered to the tenets of the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iglicki, M., Khoury, M., Donato, L. et al. Comparison of subretinal aflibercept vs ranibizumab vs bevacizumab in the context of PPV, pneumatic displacement with subretinal air and subretinal tPA in naïve submacular haemorrhage secondary to nAMD. “The Submarine Study”. Eye 38, 292–296 (2024). https://doi.org/10.1038/s41433-023-02676-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02676-9
This article is cited by
-
Safety, structure and function five years after hESC-RPE patch transplantation in acute neovascular AMD with submacular haemorrhage
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)