Abstract
Objectives
To describe the application of stereotactic guidance, its preoperative workup, and limitations, if any, during orbital decompression surgery of the lateral orbital wall for thyroid-associated orbitopathy (TAO).
Methods
Case-controlled series of seven patients who underwent stereotactic-guided surgical navigation during external approach balanced orbital decompression with maximal debulking of the lateral wall.
Results
A progressive increase in debulking of the greater wing of sphenoid and exposure of dura was noted in the series. The average proptosis reduction was 9.36 mm. No complications were encountered during any of the cases, nor was there any new onset postoperative diplopia. In all cases, exposure of dura was planned and did not present as a surprise. Stereotactic setup added 10 min to preparation time.
Conclusions
Stereotactic guidance improves anatomic localization and precision during orbital decompression, increasing confidence, and reducing surgical stress. The ability to accurately determine the maximal limits of decompression real time, while confirming depth of bone removal, offers the possibility of reduced risk of iatrogenic injury. Stereotactic navigation allows for improved intraoperative localization that may improve the ability to maximally decompress the lateral wall, including the zygoma, orbital roof, and trigone, and extending towards the optic nerve with exposure of dura through smaller incisions.
Similar content being viewed by others
Introduction
Surgical decompression of the orbit is an established treatment of thyroid-associated orbitopathy (TAO) for progressive proptosis, optic neuropathy, raised IOP, or aaesthetic rehabilitation not responding to medical management.1, 2, 3, 4, 5, 6 The mainstay of treatment has been two wall decompression originally described by Walsh and Ogura4 in 1957, modified into an endoscopic formulation by Kennedy in 1990.7 Although inferomedial orbital decompression has afforded good results in terms of proptosis reduction, it accrues a large risk of postoperative diplopia, with rates of postoperative motility imbalance as high as 80%.8 In fact, even with the creation of a strut at the ethmoid–maxillary junction, two wall decompression still carries such a high risk of postoperative diplopia9, 10 that some surgeons do not even consider it to be a complication.11
Alternative techniques have been sought in an attempt to decrease decompression-induced diplopia;1 Graham et al12 suggested a balanced decompression that requires lateralization of the outfractured lateral wall, whereas the lateral orbital wall has recently been suggested as the region of first choice for orbital decompression as it provides a low risk of consecutive diplopia or severe complications, such as cerebrospinal fluid leak.13 Despite the wide variety of innovative approaches designed by multidisciplinary teams, orbital decompression remains a technically challenging procedure where the goal of proptosis reduction competes with postoperative diplopia and cosmetic requests of an increasingly demanding patient population.
Stereotactic localization is typically employed during neurosurgery to assist with localization of soft tissue structures.14 Stereotactic-guided orbital bony surgery differs slightly from that of neurosurgery; although soft tissues may move during surgery, the bony structures of the orbit do not move, and maintain their alignment with the reference plane, allowing precise localization of incisions into bone during surgery. The use of image guidance has just recently been reported for endoscopic orbital decompression however the technique was poorly described.15 This pilot study describes our experience of the application of stereotaxis to conventional small incision orbital decompression surgery to assist with localization of the limits of the bony lateral orbital wall. Specifically, we: (i) outline the principles of stereotactic guidance applied to orbital decompression surgery; (ii) develop the protocol for application of fiducial markers for stereotactic navigation of the orbit to achieve accurate orbital navigation; (iii) report the reduction in proptosis and surgical outcomes using stereotactic navigation, and compare with results from conventional decompression from the literature; and (iv) report our experience and impressions with the application of stereotactic navigation to orbital surgery.
Materials and methods
Principles of stereotactic guidance
In principle, stereotaxis involves the three-dimensional computerized localization of anatomical structures using an external guidance system. Preoperative imaging is reconstructed into a volume that can be reformatted into any plane in image space. The image space refers to this reformatted volumetric representation of the patient that is stored in the computer, in comparison with the patient space, which is the real surgical space. To use the images of the image space, both intraoperatively and in real time, the image space is correlated with the surgical space of the patient. The correlation of these points is termed as registration. The accuracy of image guidance depends on the registration, and the computer gives an estimate of accuracy called the predicted accuracy. This is carried out using a reference plane that is rigidly fixed to the skull before and during surgery. Landmarks are chosen in three-dimensional image space, and matched with landmarks in surgical space. These landmarks may be attached directly to the skull through titanium screws, or more conventionally, by adhesive markers termed as ‘fiducial markers’ that adhere to the skin. Ideally, these markers should be applied to skin that is unlikely to move after application or during the registration process, as the less the movement, the greater the accuracy. Once the location of these reference points is known, that of other structures in image space may be calculated with reference to these points. The localization system comprises a workstation and a dual infra-red camera mounted to a pole. An optical reference arc is attached to the head using pinpoint fixation once the head has been immobilized. The location of this arc is registered with the workstation, and then the location of the reference points with respect to the arc is registered.
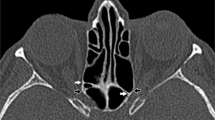
As there are no manufacturer or published guidelines on placement of orbital fiducial markers, we undertook several cases with varying placement of fudicial markers to determine the most reliable placement. We now use a modified ENT protocol for the application of these markers (see Figure 1) that achieves a 1-mm zone of accuracy around the orbit. Surgery was undertaken through a transconjunctival external approach to the medial wall, and a lateral skin crease incision to the lateral wall. Bone removal was carried out using high-speed surgical drills.
Case series
This is a case controlled series of seven patients who underwent a balanced medial and lateral wall decompression using stereotactic navigation. All patients had stable TAO, whose indication for surgery included proptosis, intraocular pressure control, or cosmesis. Information obtained included preoperative and postoperative Hertel measurements, age, sex, the side of surgery, diplopia history, visual acuity, surgical complications, and follow-up period.
In this study, the lateral wall decompression is considered in two parts, the lateral and deep lateral walls. The lateral wall includes the zygomatic bone and lower aspect of the frontal bone, and the deep lateral wall is the orbital face of the greater wing of sphenoid posteromedially and the trigone posterolaterally. During surgery, a lateral decompression was undertaken through a lateral skin crease incision. In all cases, dissection was carried out to the lateral orbital rim with elevation of the periorbita vertically from the roof to the floor, and posteriorly to within 10 mm of the optic nerve as indicated by stereotactic guidance, using the Stealth Station (Medtronic, CO, USA) and cranial software. Complete removal of the lateral wall from the posterior orbital rim to the temporal fossa was undertaken using a high speed 4-mm diameter cutting burr, and completed with a diamond burrs of 4 and 3 mm diameter, exposing dura. The superior limits of decompression were noted to vary depending on bony anatomy, and in each case, the safe maximal superior limit was determined using surgical navigation. We aimed to remove bone if it was 1.5 mm in thickness or greater as determined by surgical navigation. In the surgical plane between the superior and inferior orbital fissure, surgical navigation was used to carefully determine the maximal posteromedial limits of decompression. At completion of bone removal, the periorbita was split from posterior to anterior, and retroconal fat was repositioned temporally to fill the space created. In all cases, medial wall decompression was undertaken through a transcaruncular approach as conventionally described by Perry et al.16
Results
A total of seven patients underwent monolateral stereotactic-guided orbital decompression. The Follow-up interval ranged from 9 months to 3 years; measurement parameters are reported at 6 months after surgery. Proptosis was reduced on average by 9.36 mm (range: 7–12 mm). Reduction in proptosis was noted to improve with each sequential decompression. No intraoperative or postoperative complications were observed (see Table 1). No patient suffered new onset postoperative diplopia, and there was no change in extraocular muscle motility postoperatively. The best-corrected visual acuity remained unchanged. All patients were satisfied with their results. No patient suffered other neurological deficits.
Discussion
Although successful orbital decompression relies as much on knowledge of the disease progress of TAO and its staging, surgical planning is critical to ensure that an appropriate level of decompression is achieved with minimal collateral damage.17 There has been an increase in the literature over the recent years describing the technique of balanced decompression through a transconjunctival and canthotomy, and most recently, endoscopic approach.12, 18 This combination of lateral and medial wall decompression achieves a volume reduction of approximately 5 mm,18 with the option to add more walls and/or fat decompression. Complete removal of the lateral wall alone is an accepted approach advocated by Goldberg et al,18 and only in cases requiring maximal proptosis, reduction or cases of severe compressive optic neuropathy is the medial wall decompressed.19 Variations are described; some authors advocate the removal of the lateral, without replacement of the frontal process of zygoma,17 whereas other techniques add orbital space by reducing the lateral space to do valgus rotation, thereby retaining the frontal process, and expanding the space.20 Although many techniques of decompression have been advocated, the surgeon should retain the option to open up any orbital wall as patients present with varying preoperative conditions in regard to optic neuropathy, diplopia, cosmesis, and degree of proptosis.7
In the demand for small incision surgery in modern day surgical era, recent trends have been towards both anaesthetically insignificant incisions and endoscopic approaches, although concurrently reducing the incidence of postoperative diplopia and iatrogenic injury.6, 7, 8, 9, 10, 11, 12, 13, 14, 15 With the advent of smaller incision orbital surgery, perspective and localization becomes increasingly difficult and may pose a limitation against maximal decompression. However, even when good exposure of the floor is obtained through the transorbital approach reported by McCord21 and Anderson and Linberg,22 the medial wall view can occasionally be difficult, particularly with bleeding in the crucial area of the orbital apex, with potentially unpleasant consequences for both clinicians and patients. In fact, although balanced and lateral wall decompression has been advocated to minimize the incidence of postoperative ocular imbalance with diplopia, a not insignificant rate of complications still occur (see Table 2).
Stereotactic navigation has been investigated as a means of reducing postoperative diplopia and iatrogenic injury in endoscopic balanced decompression, and described for the endoscopic nasal component.15 The advantages of deep bony decompression posterior to the lateral orbital rim are (i) less postoperative morbidity; (ii) when approached from the internal aspect, temporalis muscle trauma is minimized, reducing the risks of secondary muscle atrophy, potential for haemorrhage and chewing difficulty; and (iii) it is a faster technique.33 We believe that maximizing bone removal allows for less need for orbital fat removal, and minimizing direct injury to the lateral recti muscle and the potential for nerve damage. This may be the reason for no new onset diplopia using this technique.
We believe that stereotactic navigation is specifically advantageous in decompression of the lateral and deep lateral wall as the surgical limits of lateral wall decompression vary in the literature, and its limits in three planes are not universally agreed. Furthermore, it is our experience that the posterior limits of deep lateral wall decompression are difficult to define intraoperatively, and difficult to standardize. This is complicated by the limited access for multiple surgical instruments required to remove this bone, as well as bleeding that occurs as the trigone is removed. Despite the above, we believe that a maximum lateral decompression is desirable. The contribution of the posterolateral wall decompression for exophthalmos reduction has been recently shown to be 2.3 mm, as part of a coronal approach, 3-wall decompression without increasing the risk of consecutive diplopia.33 The ideal bone removal of the lateral wall would include both lateral and deep lateral walls, extending from the roof to the floor, with maximal debulking of the trigone. However, as both bone thickness and anatomy varies between patients, the exact and appropriate removal of any part of the orbit is likely to vary between cases. Sires34 has tried to circumvent this problem by using sequential intraoperative CT imaging to identify the limits of decompression and confirm globe retroplacement. With stereotactic navigation, the limits of decompression in image space are visible on a computer monitor real time, avoiding unnecessary CT imaging and radiation to the patient. Stereotactic control may allow for bone removal to approach this ideal maximum, and thereby improve predictability of retroplacement of the globe. Although the initial use of this technology seems daunting, and time and effort is required in its adoption, operating time and costs increase at our institution by only 10 min and $500, similar to that seen in sinus surgery using stereotaxis.35 A complete list of the benefits of stereotactic-guided decompression can be found in Table 3.
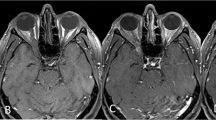
It was our intention to completely remove the trigone, and we were able to achieve this with surgical navigation. Reduction in proptosis was noted to improve with each sequential decompression that is probably due to increased bone removal from the deep lateral wall of the orbit. We did note intraoperatively that the volume of the bony lateral wall in this area varied between patients when the limits were explored using computer navigation. Safe exposure of the dura of the anterior and middle cranial fossa was achieved (see Figure 2) following debulking of the greater wing of the sphenoid; the exposure of dura was anticipated in each case. Although our series is limited to seven in number, we believe the lack of complications is due in part to the additional visualization and excellent exposure to the deep lateral wall provided by stereotactic guidance. Removal of the posterolateral wall in the trigone region and its posteromedial extension is a potential danger to the region close to the middle cranial fossa. As it was our aim to maximize volume expansion one would anticipate a complication rate similar to that of other studies. We believe that the ability to accurately localize the surgical dissection plane with navigation technology assists with surgical safety. It quickly became evident that surgical navigation provided objective control in removing bone from the deep lateral wall, which we would otherwise have left in place. This is reflected by the sequential improvement in proptosis reduction with each case. Figure 3 illustrates the difference in bone removal using stereotactic navigation in a patient who underwent sequential bilateral lateral orbital wall decompression, using conventional surgery on the right by another ophthalmic plastic reconstructive surgeon experienced in orbital decompression, and stereotactic surgery on the left by the senior author.
In conclusion, we have described the preoperative planning and the application of surgical navigation to lateral and deep lateral wall orbital decompression, and the outcomes of a pilot study of seven patients undergoing balanced orbital decompression using stereotactic intraoperative control of bone removal. In our case series, we were able to achieve an average of 9.36 mm of retroplacement using a balanced decompression with extensive removal of the lateral and deep lateral wall, including its posteromedial and posterolateral extension, under stereotactic control. It is our experience that stereotactic guidance may offer assistance to the surgeon at various stages of the bony decompression, such as determining the absolute anterior limits for initiation of bone removal, determining the absolute superior, inferior, and posterior limits of real time, and confirming the depth of bone removal, and thus may reduce the risk for any iatrogenic injuries and serious complications, such as breach of the dura mater, or even direct cerebral trauma. Computerized guidance by definition allows for a precise intraoperative assessment of bone, particularly around the region of the trigone, and may allow for maximum bone removal of the lateral and deep lateral walls through small cosmetically acceptable incisions. We continue to employ this technique.
References
Shepard KG, Levin PS, Terris DJ . Balanced orbital decompression for Grave's ophthalmopathy. Laryngoscope 1998; 108: 1648–1653.
Dollinger J . Die Druckentlastung der Augenhöhle durch Entfernung der äuβeren Orbitawand bei hochgradigem Exophthalmos (Morbus BAsedowii) und konsekutiver Hauterkrankung. Dtsch med Wschr 1911; 37: 1888–1890.
Olivari N . Transpalpebral decompression of endocrine ophthalmopathy (Graves' disease) by removal of intraorbital fat: experience with 147 operations over 5 years. Plast Reconstr Surg 1991; 87: 627–643.
Walsh TE, Ogura JH . Tansantral orbital decompression for malignant exophthalmos. Laryngoscope 1957; 67 (6): 544–568.
Crespi J, Rodrigeuz F, Buil JA . Intraocular pressure after treatment for thyroid-associated ophthalmopathy. Arch Soc Esp Oftalmol 2007; 82: 691–696.
Kalmann R, Mourits MP . Prevalence and management of elevated intraocular pressure in patients with Graves' Orbitopathy. B J Ophthalmol 1998; 82: 754–757.
Kennedy DW, Goodstein ML, Miller NR, Zinreich SJ . Endoscopic transnasal orbital decompression. Arch Otolaryngol Head Neck Surg 1990; 116: 275–282.
De Santo LW . Transantral orbital decompression. In: Gorman CA, Waller RR, Dyer JA (eds). The Eye and Orbit in Thyroid Disease. Raven Press: New York, 1984, pp 231–251.
Garrity JA, Fatourechi V, Bergstralh EJ, Bartley GB, Beatty CW, De Santo LW et al. Results of transantral orbital decompression in 428 patients with severe Graves' ophthalmopathy. Am J Ophthalmol 1993; 116: 533–547.
Maroon JC, Kennerdell JS . Radical orbital decompression for severe dysthyroid exophthalmos. J Neursurg 1982; 56: 260–266.
Metson R, Dallow RC, Shore JW . Endoscopic orbital decompression. Laryngoscope 1994; 104: 950–957.
Graham SM, Brown CL, Carter KD, Song A, Nerad JA . Medial and lateral orbital wall surgery for balanced decompression in thyroid eye disease. Laryngoscope 2003; 113 (7): 1206–1209.
Goldberg RA, Perry JD, Hortaleza V, Tong JT . Strabismus after balanced medial plus lateral wall vs lateral wall only orbital decompression for dysthyroid orbitopathy. Ophthal Plast Reconstr Surg 2000; 16 (4): 271–277.
Apuzzo ML, Sabshin JK . Computed topographic guidance stereotaxis in the management of intracranial mass lesions. Neurosurgery 1983; 12: 277–285.
Dubin MR, Tabaee A, Scruggs JT, Kazim M, Close LG . Image-guided endoscopic orbital decompression for Graves' orbitopathy. Ann Otol, Rhinol Largngol 2008; 117 (3): 177–185.
Perry JD, Kadakia A, Foster JA . Transcaruncular orbital decompression for dysthyroid optic neuropathy. Ophthal Plast Reconstr Surg 2003; 19 (5): 353–358.
Rootman J, Stewart B, Goldberg RA . Orbital Surgery: A Conceptual Approach, 1st edn. Lippincott-Raven Publisher: Philadelphia, 1995, pp 353–384.
Goldberg RA, Kim AJ, Kerivan KM . The lacrimal keyhole, orbital door jamb, and basin of the inferior orbital fissure: three areas of deep bone in the lateral orbit. Arch Ophthalmol 1998; 116: 1618–1624.
Goldberg RA . Commentary on customized, single-incision, three-wall orbital decompression. Opthal Plast Reconstr Surg 2005; 21 (1): 9–10.
Paridaens DA, Verhoeff K, Bouwens D, van den Bosch WA . Transconjunctival orbital decompression in Graves' ophthalmopathy: lateral wall approach ab interno. Br J Ophthalmol 2000; 84: 775–781.
McCord CD . Orbital decompression for Graves' disease, exposure through lateral canthal and inferior fornix incision. Ophthalmology 1981; 88: 533–541.
Anderson RL, Linberg JV . Transorbital approach to decompression in Graves' disease. Arch Ophthalmol 1981; 99: 120–124.
Warren JD, Spector G, Burde R . Long-term follow-up and recent observations on 305 cases of orbital decompression for dysthyroid orbitopathy. Laryngoscope 1989; 99: 35–40.
Tallstedt L, Papatziamos G, Lundblad L, Anggard A . Results of transantral orbital decompression in patients with thyroid-associated ophthalmopathy. Acta Ophthalmol Scand 2000; 78: 206–210.
Wright ED, Davidson J, Codere F, Desrosiers M . Endoscopic orbital decompression with preservation of an inferomedial bony strut: minimization of postoperative diplopia. J Otolaryngol 1999; 28 (5): 252–256.
Stiglmayer N, Mladina R, Tomi M, Tojagi M, Juri J, Bubas N et al. Endonasal endoscopic orbital decompression in patients with Graves' ophthalmopathy. Croat Med J 2004; 45 (3): 318–322.
Kasperbauer JL, Hinkley L . Endoscopic orbital decompression for Graves' ophthalmopathy. Am J Rhinol 2005; 19 (6): 603–606.
Mourits PHM, Koorneef L, Wiersinga WM, Prummel MF, Berghout A, van der Gaag R . Orbital decompression for Graves' ophthalmopathy by inferomedial, by inferomedial plus lateral, and by coronal approach. Ophthalmology 1990; 97: 636–641.
Kalmann R, Mourits MP, van der Pol JP, Koornneef L . Coronal approach for rehabilitative orbital decompression in Graves' ophthalmopathy. Br J Ophthalmol 1997; 81: 41–45.
Ulualp SO, Massaro BM, Toohill RJ . Course of proptosis in patients with Graves' disease after endoscopic orbital decompression. Laryngoscope 1999; 109 (8): 1217–1222.
Leone Jr CR, Piest KL, Newman RJ . Medial and lateral wall decompression for thyroid ophthalmology. Am J Ophthalmol 1989; 108: 160–166.
Sellari-Franceschini S, Berrettini S, Santoro A, Nardi M, Mazzeo S, Bartalena L et al. Orbital decompression in Graves' ophthalmology by medial and lateral wall removal. Otolaryngol Head Neck Surg 2005; 133 (2): 185–189.
Baldeschi L, MacAndie K, Hintschich C, Wakelkamp IM, Prummel MF, Wiersinga WM . The removal of the deep lateral wall in orbital decompression: its contribution to exophthalmos reduction and influence on consecutive diplopia. Am J Ophthalmol 2005; 140 (4): 642–647.
2001 University of Washington Ophthalmology Ground Rounds by Dr Bryan Sires. Orbital decompression for thyroid eye disease using intra-operative CT. Available at: http://www.researchchannel.org/prog/displayevent.aspx?rID=2441&fID=345'. (accessed on 30 August 2007).
Reardon EJ . The impact of image-guidance systems on sinus surgery. Otolaryngol Clin N Am 2005; 38: 515–525.
Acknowledgements
We thank Dr Arek Nemet from Israel for his suggestions on the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Millar, M., Maloof, A. The application of stereotactic navigation surgery to orbital decompression for thyroid-associated orbitopathy. Eye 23, 1565–1571 (2009). https://doi.org/10.1038/eye.2009.24
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2009.24
Keywords
This article is cited by
-
Deep lateral orbital decompression for Graves orbitopathy: a systematic review
International Ophthalmology (2021)
-
Practice Patterns in Orbital Decompression Surgery Among American Society of Ophthalmic Plastic and Reconstructive Surgery Members
Ophthalmology and Therapy (2019)
-
Critical Appraisal on Orbital Decompression for Thyroid Eye Disease: A Systematic Review and Literature Search
Advances in Therapy (2015)