Abstract
Background/objectives
This study aims to identify radiologically the position of the optic foramen in relation to the anterior face of the sphenoid sinus, to aid surgeons in their planning for orbital decompression.
Methods
CT scans of 100 orbits from 50 adult patients without any abnormality were assessed. Primary outcome measures included: position and measurement of the distance from the optic foramen to the anterior face of the sphenoid sinus. Secondary outcomes included: medial orbital wall length, distance from the optic foramen and the anterior face of the sphenoid sinus to the carotid prominence in the sphenoid sinus, and the thickness of bone anterior to the optic foramen.
Results
The mean location of the optic foramen was just posterior to the position of the anterior face of sphenoid sinus, with an average distance of +0.4 +/− 3.5 mm. In 54% of orbits the optic foramen was positioned posterior to the anterior face of the sphenoid sinus. The finding was symmetrical in 80% of patients.
Conclusions
Our study identifies that the optic foramen lies posterior to the anterior face of sphenoid sinus in approximately half of cases. The position may be asymmetric in 20% of individuals.
Similar content being viewed by others
Introduction
The anatomy of the orbit, in particular the orbital apex, is recognised as being complex with a number of vital structures enclosed in a tight space [1]. The optic nerve enters the orbit via the optic foramen and immediately runs through the annulus of Zinn, or the common tendinous ring [1]. Orbital decompression is performed in the management of select cases of thyroid eye disease, for which dysthyroid optic neuropathy and significant proptosis are indications [2, 3]. Orbital decompression can also be performed for a variety of other aetiologies including orbital haemorrhage, benign, malignant and metastatic tumours [4].
Traditional approaches to decompress the orbit are via an external approach, with newer approaches to access the orbit via a transnasal route becoming more widely described as endoscopic surgical techniques evolve [2, 3, 5]. In select patients, in order to achieve maximal decompression, surgeons may elect to decompress the orbit as far posteriorly as the optic foramen, which would include the annulus of Zinn. In some patients the annulus of Zinn may be located posterior to the anterior face of the sphenoid sinus, meaning that the surgeon will have to enter the sphenoid sinus and remove part of the lesser wing of sphenoid to enable this complete decompression.
Furthermore, the presence of a sphenoethmoidal air cell (Onodi cell) may also alter the relationship of the sphenoid sinus to the orbital apex. An Onodi cell is a posterior ethmoidal air cell that pneumatises either superolateral, superior or lateral to the sphenoid sinus [6]. Whilst the presence of an Onodi cell has been well documented in the literature, the prevalence and identification techniques of this anatomical variant are variably reported [7,8,9].
In this study, we aim to identify radiologically the position of the optic foramen in relation to the anterior face of the sphenoid sinus, as well as other key anatomical relationships at the orbital apex. This may aid surgeons in their planning for orbital decompression.
Methods
This retrospective study was conducted at a tertiary referral hospital in Adelaide, South Australia. The described research adhered to the tenets of the Declaration of Helsinki. Institutional review board/ethics committee approval was obtained for this study.
Computed tomography (CT) scans of the orbits and sinuses of 50 adult patients (100 orbits) were retrieved from the Picture Archiving and Communication System server at the Radiology Department between March 2019 and June 2019. Images obtained for inclusion in the study included a minimum of multi-planar (axial, coronal and sagittal) bone-window slices of the paranasal sinuses, with thin (<0.8 mm) axial slices. CT scans with any significant sinus or orbital abnormality were excluded from the study, as were patients with a history of any orbital or sinus surgery. Patients with a history of suspect trauma who were included in the study, all had CT scans which were formally reported as being normal by a consultant radiologist.
All CT scan images were reviewed and measurements taken by a single author (JA), who was trained in identifying the specific landmarks by a neuro-radiologist (SP) and an orbital surgeon (DS). All authors were involved in the design of the study. Images were simultaneously viewed in all three planes when identifying all landmarks.
The following measurements were performed.
Primary outcome
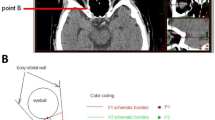
Position of optic foramen relative to anterior face of sphenoid sinus: The medial end of the optic foramen was identified on the thin axial CT slices. The superolateral aspect of the anterior face of the sphenoid sinus as it inserts into the medial orbital wall or optic canal was identified. The relative position of the optic foramen was recorded as being either anterior or posterior to the anterior face of the sphenoid. The digital calliper was used to measure the distance between these two points in the axial plane and in an antero-posterior direction. This distance was the distance of optic foramen to anterior face of sphenoid sinus (mm). Arbitrarily, a positive value was assigned when the optic foramen was located posterior to the anterior face of the sphenoid sinus, and a negative value was assigned when the optic foramen was located anterior to the anterior face of the sphenoid sinus (Figs. 1–3).
Secondary outcomes
Medial orbital wall length—axial plane (mm): The anterior lacrimal crest to the medial end of the optic foramen was measured with the digital calliper in an axial plane and recorded. The anterior lacrimal crest was chosen as a landmark for the measurement of medial orbital wall length as it is an easier to identify landmark on a CT scan, as well as it being consistent with previously published reports on this measurement.
Optic foramen to carotid prominence in sphenoid sinus—axial (mm): The medial end of the optic foramen was identified as previous. The carotid prominence in the posterior sphenoid sinus was identified and a linear measurement was taken with the digital calliper in an axial plane.
Anterior face of sphenoid sinus to carotid prominence—axial (mm): The distance between the previously identified anterior face of the sphenoid sinus to the carotid prominence in the posterior sphenoid sinus was measured in a linear fashion and in an axial plane with the digital calliper (Fig. 4).
Thickness of bone anterior to optic foramen—coronal (mm): Whilst simultaneously viewing the axial and coronal slices of the CT, the position of the medial end of the optic foramen was identified. One slice immediately anterior to the position of the optic foramen was viewed on the coronal images, and at this position the thickness of the bone at the level of the optic nerve was measured.
Statistical analysis was conducted using Microsoft Excel version 16.0. Normal distribution of data was examined using the Shapiro–Wilk test. Normal distribution of data was proven and thus mean results are presented throughout this article. Potential differences between right and left orbits were examined using a two-tailed paired t-test analysis. A simple regression analysis was used to examine the relationship of each of the measurements and other variables of interest (including age, gender and laterality). P values < 0.05 were considered statistically significant.
Results
One hundred orbits of 50 patients, 23 male and 27 female, were included. The patients were aged between 24 years and 102 years, with an average of 62.9 years. The indication for the CT scans ranged from trauma (including fall, assault, facial trauma), febrile neutropenia, seizure, ANCA vasculitis, nasal congestion and bone-marrow transplant. No patient had a history of orbital or sinus surgery or significant orbital or paranasal sinus abnormality. Regardless of the indication for the CT scan, all were formally reported as normal scans.
Position of optic foramen relative to anterior face of sphenoid sinus: In 54% of orbits the optic foramen was positioned posterior to the anterior face of the sphenoid sinus. The optic foramen was positioned anterior to the anterior face of the sphenoid sinus in 46% of orbits. The finding was symmetrical (Figs. 2 and 3) in 80% of patients and asymmetrical (Fig. 1) in 20%.
The mean location of the optic foramen was just posterior to the position of the anterior face of the sphenoid sinus, with an average distance of +0.4 +/− 3.5 mm. There was a range between −7.3 and +9.1 mm.
The results of all measurements including medial orbital wall length, optic foramen to carotid prominence in sphenoid sinus, anterior face of sphenoid sinus to carotid prominence, and thickness of bone anterior to optic foramen are summarised in Table 1.
Our data identified no statistically significant correlations between any of the measurements taken in this study with variables including age, gender or laterality. There was also no statistically significant difference between right and left orbits for any of the measurements taken in this study.
Discussion
We performed this radiological study with the aim of improving the anatomical knowledge of the orbital apex for surgeons planning orbital decompression surgery. In clinical terms, this may be beneficial in that it has the potential to influence the choice between external or endoscopic transnasal surgery in cases which may require maximal posterior orbital apex decompression. Cases where the surgeon can identify the need to enter the sphenoid sinus may be more suitable for an endoscopic transnasal orbital decompression.
We have identified that the average location of the optic foramen is just posterior to the position of the anterior face of the sphenoid sinus, with an average distance of +0.4 +/− 3.5 mm. The superolateral aspect of the anterior face of the sphenoid sinus as it inserts into the medial orbital wall or optic canal was used as the landmark in our study. The goal in identifying this portion of the anterior face of the sphenoid sinus (as opposed to the sphenoid ostium or other aspects of the anterior sphenoid) was to investigate this from the most relevant perspective of a surgeon aiming to achieve maximal posterior orbital decompression to the level of the annulus of Zinn, and thus decompress in the region of the orbital apex. Whilst in few cases the anterior face of the sphenoid sinus may be a vertical structure positioned conveniently in the coronal plane, it is apparent that the variable anatomy of this structure can introduce significant variability in measurements. The sphenoid ostium has previously been used as a landmark for the anterior face of the sphenoid sinus, and has been found to be located at almost the vertical midpoint of the anterior wall of the sphenoid sinus [10]. From the perspective of a surgeon performing surgery in the region of the orbital apex, the insertion point superolaterally may have the greatest degree of practical clinical significance.
Approximately half of the orbits in our study (54%) reveal an optic foramen position that is posterior to the anterior face of the sphenoid sinus, whilst in the other half (46%) the optic foramen is located anterior to the anterior face of the sphenoid sinus. In order to achieve the maximal orbital apical decompression (in an antero-posterior direction), about half of the orbits in this study would require surgery that involves entering the sphenoid sinus. Surgery involving the sphenoid sinus potentially comes with an increased level of risk given the numerous key anatomical structures located in close vicinity—such as the optic canal, carotid artery and skull base [10]. It is for this reason it is important for surgeons to adequately plan prior to surgery as to whether the planned orbital decompression would require surgery involving the sphenoid sinus. It may be the preference of surgeons to elect to perform endoscopic techniques to improve visualisation in these cases. Our study also identifies that for an individual patient, this may be an asymmetrical finding in 20% of cases.
The large standard deviation to a number of the measurements highlights the varied anatomy amongst individuals at the region of the orbital apex. Detailed anatomy of this region is thus far poorly documented in the scientific literature. In our study population we are unable to identify any statistically significant correlations for any measurements with patient age, gender or laterality. Perhaps with larger study populations potential correlations would be identified. Our data suggest that whilst on average the position of the optic foramen is just posterior to the position of the anterior face of the sphenoid sinus, there can be large variations between patients and also between sides in the one individual.
A Japanese study reviewed CT sinuses images of 261 patients to assess on sagittal slices the relationship of the sphenoid sinus at both the lateral side of the anterior wall and the middle of the anterior wall [9]. It was reported that 49.2% of sides were identified in which the lateral side of the anterior wall of the sphenoid sinus attached anterior to the optic canal [9]. This figure closely correlates with our study results that show 54% of sides with the anterior face of the sphenoid sinus being located anterior to the optic foramen. In another study based on normal CT scans, 48% of posterior ethmoidal cells were identified as being in contact with the ipsilateral optic canal [11]. It can be reasonably assumed that these cases would have an optic foramen sitting anterior to the anterior face of the sphenoid sinus, and translates to the portion of cases that could undergo sphenoid sinus sparing orbital decompression surgery. This result also correlates well with the findings in our study.
In a CT study of paediatric patients assessing the prevalence of Onodi cells, the relationship between the posterior ethmoid cells and the optic canal was categorised into four categories. Definitions for type 2–4 included some contact of the optic nerve canal with at least one posterior ethmoid cell, which was identified in 55.6% of cases [7]. This value would presume that the optic foramen was adjacent to a posterior ethmoid cell in these cases and the sphenoid sinus being located further posteriorly. A much earlier comparison CT anatomical study identified a posterior ethmoid cell in contact with the optic canal in only 3% of cases, however this study only assessed in the coronal plane and with 4-mm slices [12]. There is significant variability in results of the current published literature when it comes to assessment of the orbital apex and in particular the relationship with the ethmoid and sphenoid sinuses. Conclusions are often possible only by extrapolating from the data previously published and frequently are also dependent on a number of assumptions. Given that there is a lack of literature specifically assessing the key anatomical position of the optic foramen relative to the surrounding sinuses, this highlights the usefulness of our study, which primarily assesses the position of the optic foramen (and thus the annulus of Zinn) relative to the anterior face of the sphenoid sinus.
From the results of our study, the 46% of orbits in which the optic foramen sits anterior to the anterior face of sphenoid sinus, is purely an anatomical finding. There may be other factors that contribute to whether or not entry into the sphenoid sinus is necessary if the aim is to perform maximal orbital apical decompression, such as the angulation of the anterior face of the sphenoid as it inserts onto the medial wall of the orbit. Speculation on these values or criteria is beyond the scope of this study and would require further research. However, we believe that it is worth highlighting the clinical importance of these anatomical variations in preoperative planning. These will influence whether the surgeon will consider performing a sphenoidectomy or not, if the aim is to perform an orbital decompression posteriorly to the level of the annulus of Zinn. This applies to both endonasal and external approaches. Entering the sphenoid sinus does carry an increased level of surgical risk, including major vascular injury to the carotid artery.
Where maximal posterior orbital decompression is not required, such as in cases performed for aesthetic reasons, performing a sphenoidectomy and thus increasing the risk of potential major vascular injury would ordinarily be considered unnecessary. The opposite may be true for certain cases of dysthyroid optic neuropathy, however further investigation correlating optic nerve function with the extent of orbital decompression is required. Results after performing an augmented endoscopic medial orbital wall decompression for refractory dysthyroid optic neuropathy, to achieve maximal orbital apex decompression have been recently published [2], but a comparison to less extensive orbital decompression is thus far lacking.
The length of the medial orbital wall is documented in numerous articles previously published in the scientific literature. Our mean result of 38.8 +/− 2.7 mm is in keeping within the range of previously published reports [13,14,15,16].
The mean distances from the optic foramen and the anterior face of the sphenoid sinus to the carotid prominence in the posterior sphenoid sinus, of 8.6 mm and 8.7 mm, respectively, highlight the need for a sound understanding of the regional anatomy in order for a surgeon to safely perform endoscopic endonasal orbital surgery. In the literature, the distance from the sphenoid ostium to the posterior wall of the sphenoid sinus, measured by extending a line from the nasal sill to the sphenoid ostium, revealed a mean distance of almost 14 mm on CT in Japanese adult patients [10]. The sphenoid ostium was reported to be located at the vertical midpoint of the anterior wall of the sphenoid sinus, which differs significantly from the reference point used in our study. The sphenoid ostium to the nearest point on the optic canal was 15.5 mm, while the distance to the nearest point on the carotid artery was reported at 25 mm [10].
The width of the optic canal wall that separates the optic canal from the sinus cavity (either the posterior ethmoid or the sphenoid sinus) has previously been reported as between 0.9 and 1.1 mm based on normal patient CT [11]. Our measurement of the thickness of bone in a more anterior location just anterior to the optic foramen, whilst not directly comparable was similar with a mean value at 1.1 mm. The value of this measurement is in that it represents the thickness of bone needing to be breached via an endoscopic transnasal approach in order to access the periorbita to perform a maximal posterior orbital decompression at the level of the annulus of Zinn. This bone at the optic tubercle is generally thicker than the bone of the lamina anterior to it and usually requires a drill for removal.
One notable limitation of this radiological study includes that the routine acquisition of CT orbits and sinuses images often do not allow for the key landmarks studied in our study to be identifiable on a single axial CT slice. To gain these ideal views in a particular patient and on a single CT plane may require reconstruction of the images with tilting of the scan plane and thickening of the slices. It may be beneficial in the surgical planning to create such a reconstruction as a standard preoperative protocol so surgeons can get this plane without having to manually reconstruct images.
In conclusion, our radiological anatomical study primarily identifies that the position of the optic foramen lies posterior to the position of the anterior face of sphenoid sinus in approximately half of orbits (54%). In addition, this may be asymmetric in 20% of individuals. This knowledge of the anatomy of the orbital apex and its surrounding structures will aid surgeons in their planning for orbital decompression surgery.
Summary
What was known before
-
Orbital apex anatomy is complex with a number of vital structures in close vicinity.
-
Some anatomical relationships are yet to be well described, such as that of the relationship between the optic foramen and the anterior face of the sphenoid sinus.
-
Orbital decompression surgery to the orbital apex may be required in certain cases to preserve optic nerve function.
What this study adds
-
In approximately half of orbits, the optic foramen is positioned posterior to the anterior face of the sphenoid sinus.
-
Orbital decompression posteriorly to the level of the annulus of Zinn, theoretically involves entering the sphenoid sinus in such cases, potentially increasing the risk to vital surrounding structures.
-
This anatomical knowledge may influence surgeons on their choice between external and endonasal techniques for select orbital decompression cases.
References
Cornelius CP, Mayer P, Ehrenfeld M, Metzger MC. The orbits-anatomical features in view of innovative surgical methods. Facial Plast Surg. 2014;30:487–508.
Singh S, Curragh DS, Selva D. Augmented endoscopic orbital apex decompression in dysthyroid optic neuropathy. Eye. 2019;33:1613–8.
Wu W, Selva D, Bian Y, Wang X, Sun MT, Kong Q, et al. Endoscopic medial orbital fat decompression for proptosis in type 1 graves orbitopathy. Am J Ophthalmol. 2015;159:277–84.
Stokken J, Gumber D, Antisdel J, Sindwani R. Endoscopic surgery of the orbital apex: outcomes and emerging techniques. Laryngoscope. 2016;126:20–4.
Lund VJ, Larkin G, Fells P, Adams G. Orbital decompression for thyroid eye disease: a comparison of external and endoscopic techniques. J Laryngol Otol. 1997;111:1051–5.
Stammberger HR, Kennedy DW, Anatomic Terminology G. Paranasal sinuses:anatomic terminology and nomenclature. Ann Otol Rhinol Laryngol Suppl. 1995;167:7–16.
Chmielik LP, Chmielik A. The prevalence of the Onodi cell—most suitable method of CT evaluation in its detection. Int J Pediatr Otorhinolaryngol. 2017;97:202–5.
Ozdemir A, Bayar Muluk N, Asal N, Sahan MH, Inal M. Is there a relationship between Onodi cell and optic canal? Eur Arch Otorhinolaryngol. 2019;276:1057–64.
Wada K, Moriyama H, Edamatsu H, Hama T, Arai C, Kojima H, et al. Identification of Onodi cell and new classification of sphenoid sinus for endoscopic sinus surgery. Int Forum Allergy Rhinol. 2015;5:1068–76.
Enatsu K, Takasaki K, Kase K, Jinnouchi S, Kumagami H, Nakamura T, et al. Surgical anatomy of the sphenoid sinus on the CT using multiplanar reconstruction technique. Otolaryngol Head Neck Surg. 2008;138:182–6.
Bansberg SF, Harner SG, Forbes G. Relationship of the optic nerve to the paranasal sinuses as shown by computed tomography. Otolaryngol Head Neck Surg. 1987;96:331–5.
DeLano MC, Fun FY, Zinreich SJ. Relationship of the optic nerve to the posterior paranasal sinuses: a CT anatomic study. AJNR Am J Neuroradiol. 1996;17:669–75.
Akdemir G, Tekdemir I, Altin L. Transethmoidal approach to the optic canal: surgical and radiological microanatomy. Surg Neurol. 2004;62:268–74.
Abed SF, Shams P, Shen S, Adds PJ, Uddin JM. A cadaveric study of the morphometric and geometric relationships of the orbital apex. Orbit. 2011;30:72–6.
Karakas P, Bozkir MG, Oguz O. Morphometric measurements from various reference points in the orbit of male Caucasians. Surg Radio Anat. 2003;24:358–62.
Singh J, Rahman RA, Rajion ZA, Abdullah J, Mohamad I. Orbital morphometry: a computed tomography analysis. J Craniofac Surg. 2017;28:e64–70.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aujla, J.S., Curragh, D.S., Patel, S. et al. Orbital apex anatomy: relationship between the optic foramen and anterior face of sphenoid sinus — a radiological study. Eye 35, 2613–2618 (2021). https://doi.org/10.1038/s41433-020-01289-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01289-w