Credit: Stephan Schmitz/Folio Art
Not so long ago, the textbook image of the lungs was that of a sterile environment. “When I was in medical school, around 2005, literally my pathology textbook said that the normal lung is free from bacteria,” recalls Robert Dickson, a pulmonary and critical-care physician at the University of Michigan in Ann Arbor. “This was dogma for more than a century.” But over the past decade, that picture has gradually been scrubbed away as sampling of the lungs has unmasked a community of microorganisms hidden inside — albeit an unusual one.
The lung menagerie looks nothing like the microbial rainforest that thrives in the fertile gut; by comparison, the lungs are a veritable desert. “The quantity is really low — many orders of magnitude lower than the upper respiratory tract, never mind the gastrointestinal tract,” says Ronald Collman, a microbiologist and pulmonary physician at the University of Pennsylvania in Philadelphia.
The lungs’ microbial community is also notably more transient than that of the gut. The body has evolved ways to keep the lungs clean, so in healthy lungs there are few or no resident replicating bacteria. Instead, the lungs host a constant flux of microbes that mostly mirror the diverse community of the upper airways, especially around the back of the throat and the vocal cords.
But the systems that prevent the warm, wet lungs from being the perfect accommodation for bacteria can degrade. In people with chronic conditions, Dickson says, lung tissue becomes inflamed and the environment changes — mucus production increases, airway tissue swells, nutrients become more readily available to bacteria and potentially damaging strains such as Pseudomonas and Haemophilus influenzae can bloom and become resident.
Pseudomonas bacteria can cause lung infections.Credit: David M. Phillips/SPL
Most strikingly, there are signs that a shift in the lung microbiota might begin in advance of some conditions, such as chronic obstructive pulmonary disease (COPD) and lung cancer, and support their development. If proved correct, this could make lung microbes a target for intervention to prevent or delay disease. “One might be able to do respiratory microbiome therapeutics,” says Collman.
A healthy state of flux
Although it is now accepted that a healthy lung is not sterile, researchers have not yet been able to define what the microbial contents of a normal lung should be. “We don’t know yet how to best define a healthy microbiome,” says Yvonne Huang, a lung-disease specialist at the University of Michigan. She recalls that she spent two days as part of a specialist committee for the US National Academy of Sciences debating and failing to agree on such a definition in 2017.
Nature Outlook: The human microbiome
Some bacteria do seem to be common in the lungs of healthy people. “Certain microbes keep coming up,” says Stavros Garantziotis, a lung researcher at the US National Institute of Environmental Health Sciences in Durham, North Carolina. Key players include Prevotella, Streptococcus and Veillonella species, all bacterial residents of the upper airways. They probably enter the lungs through the inhalation of small droplets while people are sleeping — something that occurred in around half of a group of healthy adults in a study involving a radioactive tracer, to varying extents1.
Under typical circumstances, even these bacteria are more like regular tourists to the lung than residents — the body continually works to remove them. “Think about this dynamic community as like a train station, with people coming and going, over and over,” says Leopoldo Segal, a lung clinician at NYU Langone Health in New York City. How many microbes are visiting the lungs at a given time seems to vary from person to person and throughout an individual’s life. In a study of 49 adults with healthy lungs, Segal and his colleagues found that almost half had a relatively high load of oral microbes in their lungs2. They also found that those with high bacterial load had more infection-fighting white blood cells and pro-inflammatory molecules in their lungs.
Yvonne Huang, a lung-disease specialist at the University of Michigan in Ann Arbor, says that researchers do not yet know how best to define a healthy lung microbiome.Credit: Guowu Bian
Some evidence suggests that this immune activity could be beneficial to health. In a 2021 study, human oral bacteria were infused into the lower airways of mice, causing dysfunctional changes in the distribution of microbiota in the lungs, known as dysbiosis. This was rapidly cleared by the mice, but caused a prolonged immune response that made them less susceptible to Streptococcus pneumoniae, a cause of pneumonia3. “Benign commensals may have some beneficial roles in priming your immune system to respond better to a pathogen,” says Segal. However, aspiration of oral bacteria could also exacerbate inflammatory injury, he adds.
“There is a lung microbiome that seems to contribute to health,” says Garantziotis. Conversely, some bacteria seem to be associated with lung disease. “It is probably logical to assume certain bacteria in your lungs will predispose you to inflammation,” he says.
Disease links
Across dozens of studies and hundreds of volunteers, much the same assortment of bacteria turns up in the lungs of people with chronic conditions such as COPD and the scarring and thickening of lung tissue known as pulmonary fibrosis. The changes in quantity or type of lung microbiota are typically subtle, and not enough to be called an infection. “There’s a host of diseases where we see disruption of the normal lung microbiome, but it’s not dramatic like in infections,” says Collman. Even so, researchers are keen to know what involvement lung microbiota might have in disease. “We’re asking if a disordered microbiota is driving a dysregulated immune response that’s contributing to injury,” says Dickson.

Robert Dickson is a pulmonary and critical-care physician at the University of Michigan in Ann Arbor.Credit: Michigan Photography
Some evidence does link bacterial load in the lungs to health outcomes. “The more bacteria we find in the lungs, the worse patients do,” says Dickson. A study of more than 300 people on mechanical ventilation linked the presence of more Staphylococcus and Pseudomonas strains in the lower airways with greater inflammation and reduced survival after 30 days4. Outcomes for people who have received lung transplants also correlate with bacterial load5. “The quantity of bacteria DNA we find in the lungs of transplant patients predicts who’s going to experience rejection and ultimately die,” Dickson says.
Although the connections between lung microbiota and health are apparent, the directionality is not — do microbes in the lungs contribute to disease, or are they simply opportunist squatters taking advantage of the disease state? Certainly, disease can make the lungs more hospitable to microbe entry or replication. “Distinguishing cause from effect is really tough in human studies of COPD, because you see destruction of tissue, excess mucus production — things that may encourage bacteria overgrowth,” says Collman.
A precise timeline is arduous to follow in individuals, especially because the gold-standard procedure for sampling the lung microbiota — a bronchoscopy — is invasive and unpleasant, and therefore undertaken only when there is a compelling benefit. “The lungs are hard to sample,” says Michael Cox, a respiratory microbiome researcher at the University of Birmingham, UK. “You must go through the mouth,” he explains, which also makes contamination with oral microbiota difficult to avoid.
Could the gut give rise to alcohol addiction?
In 2016, a study in long-tailed macaques (Macaca fascicularis) became the first to examine the dynamics of the lung microbiota over time in primates6. The team, led by researchers at the University of Pittsburgh in Pennsylvania, monitored the lungs of macaques that had an HIV-like immunosuppressive infection. They found that oral bacteria progressively accumulated in the lungs, and that the animals subsequently developed COPD-like changes. “These findings suggest that changes in the lung microbiome might contribute to the development of COPD,” says Alison Morris, a pulmonary specialist at the University of Pittsburgh who worked on the study.
The true nature of the relationship between the lung microbiota and chronic lung disease probably lies somewhere in between. “I don’t think it’s a unidirectional cause and effect,” says Huang, who has studied COPD and asthma. A deteriorating lung allows more microbes to gain entry and impairs clearance mechanisms, but greater commuting of bacteria from the upper respiratory tract could also elicit a greater response from immune cells and ramp-up inflammation. “Particular culprits might be playing a part in helping move forward the inflammatory process, leading to further inflammation and damage,” Huang says.
Beyond the lungs
Emerging connections between lung microbiota and disease are not limited to COPD — or even to the lungs at all. A connection between immune cells in the lung and diseases of the brain is also coming into view. In 2022, researchers at the University of Göttingen in Germany revealed that changes to rats’ lung microbiota influenced the animals’ susceptibility to developing multiple sclerosis (MS), an autoimmune disease of the central nervous system7. It seemed that certain immune cells in the brain, known as microglia, were influenced by microbial signalling in the lungs — behaviour that the researchers described as a “remote warning system” for the brain. This insight has led some people in the field to consider whether inhaled probiotics could one day be deployed as a treatment for MS.
Evidence of a link between lung microbes and cancer is also growing. It is already widely accepted that certain gut microbes can directly increase cancer risks, a notable example being Helicobacter pylori and stomach cancer8. There are similar concerns about the Mycobacterium tuberculosis bacteria that infect the lungs and cause tuberculosis (TB). In 2009, a review of 41 studies found that the risk of developing lung cancer was significantly higher in people with a history of TB9. More than a decade later, in 2021, a study of around 20,000 people in Taiwan concluded that cancer of the gut, breast, kidney and thyroid was more likely to spread to the lungs in people who were infected with M. tuberculosis10. Researchers suggest that metabolites from bacteria could damage the DNA of lung tissue or stoke lung inflammation, creating fertile ground for tumours.
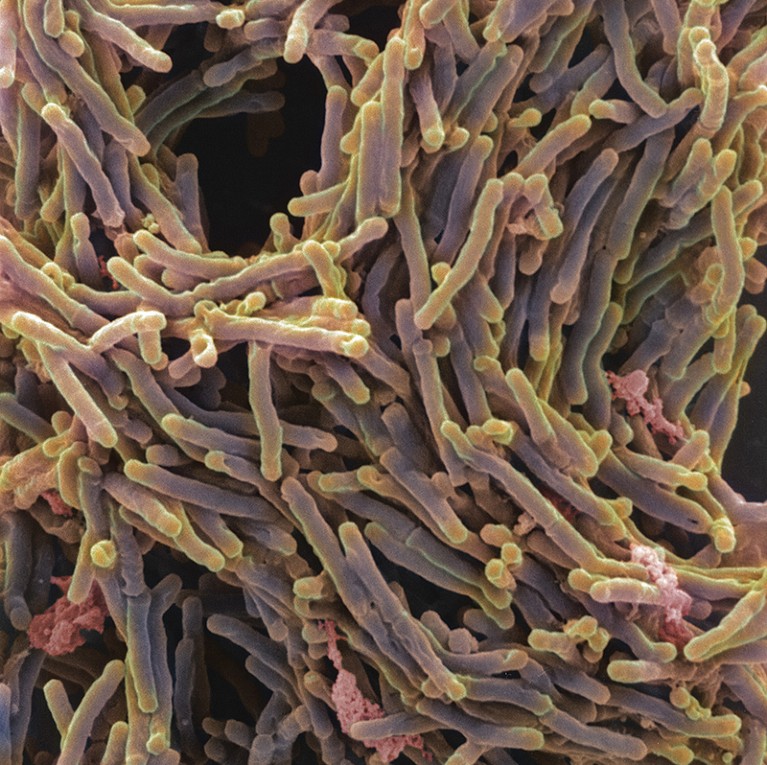
Mycobacterium tuberculosis bacteria cause the lung infection tuberculosis and have been linked to lung cancer.Credit: A. Dowsett, National Infection Service/SPL
Around the same time, Segal and his colleagues presented a study of 83 people with lung cancer in New York City that, for the first time, demonstrated that dysbiosis in the lungs affects lung tumour progression and clinical prognosis, as shown by decreased survival among people with dysbiosis and early-stage disease11. The bacteria most associated with this dysbiosis was Veillonella parvula, a microbe commonly found in the mouth, but one that is sometimes linked to infections such as gum disease. Segal and his colleagues seeded the lungs of mice with V. parvula. In mice with lung cancer, exposure to the microbe decreased survival and stoked up inflammatory markers such as IL-17. “Dysbiosis turns on an inflammatory cascade that fuels tumour progression,” says Segal. Blocking the IL-17 pathway reduced the effect of dysbiosis on tumour progression, suggesting a potential path to slowing down lung cancer.
Therapeutic aspirations
As knowledge of the lung microbiota improves, medical researchers are turning their attention to potential practical applications, such as intervening to maintain or restore lung health.
One approach to dealing with dysbiosis could be to get rid of unwanted microbes using targeted antibiotics — either in the lungs directly, or in the upper airways. “You might want to target the upper airway microbiome in disease, because that is the source of some of these microbes that reach the lungs,” says Segal. This could be especially beneficial to people who happen to aspirate more bacteria into their lungs than is considered typical, and who are therefore vulnerable to dysbiosis.
However, the effect of such an intervention can be unpredictable. In one trial that gave erythromycin — an antibiotic used to treat inflammatory airway disease — to people with lung damage, it was found that in some individuals the drug served only to replace one lot of microbes with a strain of Pseudomonas aeruginosa that was more resistant to antibiotics12.
Another strategy could be to airdrop a healthier microbial community into the lower airways, to displace unwanted residents. “The notion of a therapeutic respiratory tract repopulation is, I think, plausible,” says Collman. Which strains and in what proportions is unknown, however. “I don’t think we are quite at the mechanistic understanding for the next phase of trying to develop therapeutic approaches,” Collman says.
Some researchers are looking at lung microbiota in a different way — not as a target for therapy itself, but as a source of information that could guide the selection of existing therapies. For example, Huang’s group has found differences in the airway or lung microbiota of people who do and do not respond to inhaled steroids — drugs that are commonly prescribed for people with COPD and asthma13. She wants to further investigate what nuances in the microbiota might affect how well people respond to treatments.
“Ultimately, we need randomized controlled trials, where we ask if variation in the microbiome explains differential benefits of therapies,” says Dickson. Trials of this nature could not only help to steer treatment decisions, but also help to answer the question of directionality that plagues the studies of the links between the lung microbiota and disease. “If we can show that differential treatments work or don’t work based on your lung microbiota,” Dickson says, “that would be the most compelling argument for causality.”

 Could the gut give rise to alcohol addiction?
Could the gut give rise to alcohol addiction?
 Nature Outlook: The human microbiome
Nature Outlook: The human microbiome