Abstract
p28GANK (also known as PSMD10, p28 and gankyrin) is an ankyrin repeat anti-apoptotic oncoprotein that is commonly overexpressed in hepatocellular carcinomas and increases the degradation of p53 and Rb. NF-κB (nuclear factor-κB) is known to be sequestered in the cytoplasm by IκB (inhibitor of NF-κB) proteins 1, 2, but much less is known about the cytoplasmic retention of NF-κB by other cellular proteins. Here we show that p28GANK inhibits NF-κB activity. As a nuclear-cytoplasmic shuttling protein, p28GANK directly binds to NF-κB/RelA and exports RelA from nucleus through a chromosomal region maintenance-1 (CRM-1) dependent pathway, which results in the cytoplasmic retention of NF-κB/RelA. We demonstrate that all the ankyrin repeats of p28GANK are required for the interaction with RelA and that the N terminus of p28GANK, which contains the nuclear export sequence (NES), is responsible for suppressing NF-κB/RelA nuclear translocation. These results suggest that overexpression of p28GANK prevents the nuclear localization and inhibits the activity of NF-κB/RelA.
Similar content being viewed by others
Introduction
The oncoprotein p28GANK, containing five complete ankyrin repeats, is overexpressed in most hepatocellular carcinomas (HCCs) 3, and interacts with the CDK4 and S6b/TBP7 ATPases of the PA700/19S regulatory complex of the 26S proteasome, increasing the degradation of the retinoblastoma gene product 4, 5. p28GANK also directly binds to MDM2, which increases the p53-MDM2 association, inducing ubiquitylation and degradation of p53, and subsequently suppressing the pro-apoptotic signaling pathway mediated by p53 6. Our previous results have demonstrated that the significant downregulation of p28GANK in HCC cells by adenovirus-delivered siRNA targeting p28GANK (Adsip28GANK) inhibits cancer cell growth, and eventually results in apoptosis in vitro and in vivo 7. However, the molecular mechanisms underlying the anti-apoptotic function of p28GANK have not yet been fully elucidated.
NF-κB transcription factors commonly function as homo- or heterodimers that are formed from the multigene family of RelA (p65), RelB, c-Rel, NF-κB1 (p50/p105) and NF-κB2 (p52/p100) 8, 9. Members of the NF-κB family are characterized by the presence of a Rel homology domain, which contains a nuclear localization sequence (NLS) and is involved in sequence-specific DNA binding, dimerization and interaction with the inhibitory IκB proteins. Dimeric NF-κB complexes are linked with specific responses to different stimuli and have differential effects on transcription. NF-κB is predominantly found in the cytoplasm in an inactive form bound to the members of the IκB family 10, 11. NF-κB has major roles in the inducible expression of a large number of genes that are involved in inflammation, host defense, cell survival and proliferation. In particular, the RelA NF-κB subunit is associated with being a regulator of apoptosis 12, 13. rela knockout mice die in utero as a result of massive liver apoptosis 14. Whereas NF-κB is most commonly involved in suppressing apoptosis, it can promote programmed cell death in response to certain death-inducing signals and in certain cell types 12, 15, 16, 17. It was also shown to be indispensable for p53-dependent apoptosis 18. Given that p28GANK can negatively regulate p53-induced apoptosis, we initiated a series of experiments in this study that were designed to define the potential relationship between p28GANK and NF-κB activity.
We found that downregulation of p28GANK by siRNA increases the basal NF-κB transcriptional and DNA-binding activities, whereas overexpression of p28GANK reduces them. We present a model in which p28GANK, as a nuclear-cytoplasmic shuttling protein, associates directly with RelA to alter NF-κB nuclear translocation. Structural studies reveal that this change in NF-κB translocation is related to the nuclear export sequence (NES) of p28GANK.
Materials and Methods
Cell culture and transfection
HepG2, Hep3B, 293T, 293A and HEK293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM, GIBCO), supplemented with 10% fetal bovine serum (GIBCO). HeLa cells were cultured in RPMI medium 1640 (GIBCO), supplemented with 10% newborn calf serum (GIBCO). The protocol used for the production and application of Adsip28GANK or AdsiGFP has been previously described 7.
Reagents and plasmids
The transfection plasmids used were the pNF-κB-Luc vector, which was obtained from Clontech, and the SV40-Renilla vector, which was obtained from Sigma. Anti-gankyrin, RelA, p50 and IκBα antibodies were obtained from Santa Cruz, anti-Flag and β-actin antibodies were obtained from Sigma, anti-HA tag and Myc tag were obtained from Cell Signaling Technology. The whole cDNA sequence of p28GANK containing HA-tag (from pCMV-HA-p28GANK) was cloned into the pCDNA-3.1A-myc vector, which is used as the template for construction of a series of p28GANK deletion mutants. The mutant deleting the N-terminus (amino acids 1-38) of p28GANK (dN) was devoid of the HA-tag which was fused with the initial 38 amino acids of p28GANK.
Luciferase reporter assays
Cells were infected with 15 MOI (multiplicity of infection) Adsip28GANK or AdsiGFP 24 h before luciferase activity was measured. Cells were then transfected with 100 ng pNF-κB-luc and 1 ng SV40-Renilla using jetPEI (Polyplus transfection), and stimulated with TNF-α (30 ng/ml, R&D). The effects of p28GANK overexpression on NF-κB activity were analyzed during co-transfection with the reporter plasmids and increasing amounts of the p28GANK plasmids as above, and reporter activity was measured 24 h after transfection.
Electrophoretic mobility shift assay (EMSA)
The comparability of the various nuclear extracts was assessed in an EMSA using a Nuclear-Cytoplasmic Extract Kit (Pierce), according to the manufacturer's protocols. The probe containing the κB DNA-binding consensus oligonucleotide (5′-AGTTGAGGGGACTTTCCCAGGC-3′) was radiolabeled with biotin (Sangon, China). Nuclear protein extracts (10 μg) were incubated with the κB-biotin-labeled probe and 1 μg poly dI:dC (Promega) for 20 min at room temperature in a binding reaction buffer (10 mM Tris-HCl, pH 7.5, 10 mM KCl, 5 mM MgCl2, 1 mM DTT, 5% glycerol). The DNA-protein complexes were resolved on 6% 0.5 × TBE polyacrylamide mini-gels, according to the manufacturer's protocols (Pierce).
Immunofluorescence staining and confocal microscopy analysis
Cells were re-plated on chamber slides before transfection in the presence or absence of leptomycin B (LMB) (6 ng/ml, 2 h) from Merk. When cultured to 60-80% confluence, cells that were incubated with anti-RelA antibody conjugated to CY3 (Invitrogen) for immunofluorescence and confocal microscopic (Olympus) assay. Chromosomal DNA was visualized by staining with blue-fluorescent 4′,6′-diamidino-2-phenylindole (DAPI, Molecular Probes).
Nuclear protein extraction, immunoblotting and immunoprecipitation analyses
The Nuclear Protein Extraction Kit (from Pierce) was used according to the manufacturer's recommendations for nuclear protein extraction. Extraction of cellular proteins was carried out according to the protocol described by Upstate Biotechnology and resolved on 8-18% Tris-tricine SDS-PAGE gradient gels. Immunoprecipitation was performed as previously described 27. To analyze the interaction between endogenous p28GANK and endogenous RelA, cells were lysed and immunoprecipitated with anti-gankryin or anti-RelA antibody immobilized to the AminoLink® Plus Coupling Gel (Pierce), followed by immunoblotting.
Results
p28GANK regulates NF-κB activity
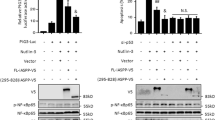
Our earlier studies have demonstrated that attenuation of p28GANK in HCC induces apoptosis 7. In an attempt to study whether the suppression of p28GANK affects the activity of endogenous NF-κB/RelA, three tumor cell lines (HepG2, Hep3B and HeLa) that overexpress p28GANK were used. After infection with Adsip28GANK, and compared with the control AdsiGFP (adenovirus-delivered siRNA targeting the green fluorescent protein), levels of p28GANK protein were significantly reduced in these tumor cells (Figure 1A). After transfection with an NF-κB-luciferase reporter gene, p28GANK knockdown led to the obvious enhancement of the basal level NF-κB transcriptional activity throughout the entire 72 h of the experiment. Tumor necrosis factor-α (TNF-α) stimulation caused further elevation of NF-κB activity, even after p28GANK knockdown (Figure 1B and data not shown). EMSA results also showed that, as expected, treatment with TNF-α increased the amount of nuclear NF-κB bound to the DNA probe. Unlike AdsiGFP-treated or mock cells, depletion of p28GANK in HepG2 tumor cells by Adsip28GANK enhanced NF-κB DNA-binding activity in a dose-dependent manner, but did not alter the binding of the constitutively expressed Sp1 transcription factor (Figure 1C). Next, we examined the effect of p28GANK overexpression on NF-κB activity. p28GANK dose-dependently suppressed the basal or TNF-α induced NF-κB activity in 293T cells (Figure 1D), but did not affect AP-1 activity (data not shown). EMSA analysis showed that p28GANK also diminished NF-κB DNA-binding activity, but did not affect Sp-1 DNA-binding activity (Figure 1E), indicating that p28GANK itself suppresses both the transcriptional and DNA-binding activity of NF-κB. To characterize the composition of the DNA-bound NF-κB complexes in the presence of the suppression by p28GANK, we performed supershift analysis with NF-κB antibodies. These NF-κB complexes were identified as heterodimers of RelA and p50, based on the ability of anti-RelA or anti-p50 to recognize the complexes (data not shown). Taken together, these results indicate that p28GANK can regulate NF-κB transcriptional and DNA-binding activities.
p28GANK inhibits NF-κB activity. (A) p28GANK protein levels in HepG2 cells infected with Adsip28GANK or AdsiGFP (negative control), respectively, were analyzed by western blot analysis using antibodies as indicated. (B) Decreased p28GANK expression induced NF-κB activity. HepG2 and Hep3B cells were infected with Adsip28GANK or AdsiGFP. Cells were or were not stimulated with TNF-α (30 ng/ml). Here, luciferase reporter results only represent the 48-h time point after infection. The average value from three independent transfections (± S.D.) is shown. (C) p28GANK knockdown increased NF-κB DNA-binding activity. Nuclear extracts from the Adsip28GANK- or AdsiGFP-infected HepG2 cells were assessed in an EMSA with a biotin-labeled NF-κB consensus oligonucleotide (N.S., non-specific shift). Expression levels of the nuclear and total cell proteins were analyzed with the indicated antibodies. Comparability of the various nuclear extracts was verified by EMSA with a biotin-labeled Sp1 probe. (D) Effects on NF-κB basal and induced activity by TNF-α stimulation. 293T cells were co-transfected with an NF-κB luciferase reporter with HA-tagged p28GANK or an empty vector. Cells were stimulated with TNF-α for 5 h before cells lysis, and then luciferase activity was determined. (E) p28GANK overexpression decreased NF-κB DNA-binding activity. 293T cells were co-transfected with RelA and Myc-tagged IκBα mutant (mut), HA-tagged p28GANK or an empty vector as indicated. Nuclear extracts were analyzed by EMSA. Expression levels of the nuclear and total extracts were analyzed by immunoblotting. The position of the NF-κB-specific complex is indicated. Comparability of the various nuclear extracts was verified by EMSA with a biotin-labeled Sp1 probe.
p28GANK alters NF-κB nuclear translocation
As p28GANK suppression did not affect the amount of total RelA (Figure 1C), we next examined whether p28GANK affects NF-κB localization. First, we monitored the intracellular trafficking of endogenous RelA by immunostaining. As shown, constitutive RelA was diffusely present in the cytoplasm and nucleus in HeLa cells (Figure 2A). RelA increasingly translocated into the nucleus after p28GANK knockdown, whereas no such change was noted in AdsiGFP-treated cells. When 293A cells were transfected with the plasmid expressing RelA, RelA accumulated in the nucleus (Figure 2B, top). However, after co-transfection with the p28GANK plasmid, RelA was sequestered in the cytoplasm (Figure 2B, bottom). Consistent with this observation, overexpressing p28GANK dose-dependently decreased the amount of nuclear RelA in 293T cells (Figure 2C). These results raise the possibility that p28GANK, like the IκBα protein, can enter the nucleus and displace NF-κB from its DNA-binding sites, and then transport NF-κB back to the cytoplasm, thereby carrying out a post-induction repression of NF-κB function. Those results further support our conclusion that p28GANK is a regulator of NF-κB activity.
p28GANK affects RelA/NF-κB nuclear translocation. (A) Increased RelA in the nucleus induced by p28GANK depletion. Images of endogenous RelA localization were analyzed by immunostaining the mock or infected HeLa cells with AdsiGFP or Adsip28GANK. (B) Decreased RelA in the nucleus induced by increased p28GANK expression. 293A cells were transfected with a RelA plasmid or in combination with GFP-p28GANK and were analyzed by immunostaining. (C) Decreased nuclear RelA induced by p28GANK. 293T cells were co-transfected with RelA and HA-tagged p28GANK or an empty vector. The nuclear extracts and whole lysates were immunoblotted with the indicated antibodies.
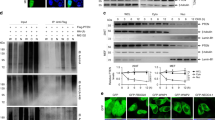
p28GANK interacts directly with RelA and accelerates nuclear export of RelA through a chromosomal region maintenance-1 (CRM1)-dependent pathway
Because no complex formation between p28GANK and IκBα was detected by the immunoprecipitation assay (data not shown), we suspected that p28GANK might interact directly with NF-κB/RelA. Confocal microscopy analysis of 293A cells revealed that RelA is sequestered in the cytoplasm and colocalized with GFP-p28GANK (Figure 3A, top). In the presence of LMB, a nuclear export inhibitor that disrupts the interaction between CRM1/exportin 1 and the nuclear export signal 19, both GFP-p28GANK and RelA colocalized mainly in the nucleus (Figure 3A, bottom). These observations illustrate that p28GANK, acting as a nuclear-cytoplasmic shuttling protein, forms a complex with NF-κB and promotes NF-κB export from the nucleus to the cytoplasm through a CRM-1-dependent pathway.
p28GANK interacts directly with RelA. (A) p28GANK colocalized with RelA in the cell. 293A cells were transfected with RelA plasmid or in combination with 1 μg/well (top panel) or 2 μg/well (middle panel) GFP-p28GANK. They were then treated with or without LMB and were analyzed by confocal microscopy after staining with anti-RelA antibody, secondary antibody and DAPI. Green, red and blue signals represent the localization of p28GANK, RelA and the nucleus. Yellow signals (merge) indicate colocalization of p28GANK and RelA. (B) Interaction of overexpressed Myc-tagged p28GANK with Flag-tagged RelA in vivo. HEK293 cells were co-transfected as indicated, and immunoprecipitated using Flag or Myc-tagged antibodies from cells. Immunoprecipitates (IPs) were immunoblotted for Myc-tagged p28GANK or Flag-tagged RelA. (C) Interaction of endogenous p28GANK with RelA in vivo. HepG2 cells were immunoprecipitated using p28GANK(lane 2) or RelA (lane 5) antibodies, and anti-mouse IgG as a negative control (lanes 1 and 4). IPs were immunoblotted for endogenous RelA (lanes 1 and 2) or for endogenous p28GANK(lanes 4 and 5) and whole-cell extracts for p28GANK or RelA as indicated.
Furthermore, co-immunoprecipitation of Flag-tagged RelA and Myc-tagged p28GANK showed the physical interactions between these two proteins in HEK293 cells (Figure 3B). The association between endogenous p28GANK and RelA was also observed in HepG2 cells (Figure 3C). Taken together, these data demonstrate that p28GANK interacts directly with RelA in vivo.
Molecular mechanisms underlying p28GANK-mediated nuclear export of RelA
It has been confirmed that the IκBα protein retains NF-κB in the cytoplasm by masking one of the two NLSs in NF-κB dimers 20, 21. To determine whether the NLS of RelA is required for p28GANK binding, we constructed a series of mutants of RelA (Figure 4A) and carried out binding assays in vivo. Analysis of various mutant RelA proteins showed that the p28GANK-interacting domain resides in residues 1-296 of RelA (Figure 4B), suggesting that the NLS of RelA is not required for the interaction. Interestingly, no interaction between p28GANK and the mutant containing the N-terminal 312 residues of RelA was observed. The reason for this difference is not known and was not investigated further in this study.
Molecular mechanisms of p28GANK alter RelA nuclear translocation. (A) Schematic representation of RelA (full) and its mutants, all fused with Flag-tag. Black boxes, nuclear localization signal (NLS); shaded boxes, Rel homology domain. (B) Interaction of overexpressed Myc-tagged p28GANK with Flag-tagged RelA mutants in vivo. HEK293 cells co-transfected with myc-tagged p28GANK and Flag-tagged deletion mutants of RelA (del-1 to del-4) were immunoprecipitated and immunoblotted using Myc or/and Flag antibody. (C) Schematic representation of p28GANK and its mutants with each of the ankyrin repeats (dA1 to dA5), the N terminus (dN) or the C terminus (dC) deleted, all fused with Myc-tag. (D) Interaction of overexpressed Flag-tagged RelA with Myc-tagged p28GANK mutants in vivo. HEK293 cells co-transfected with Flag-tagged RelA and Myc-tagged deletion mutants of p28GANK were immunoprecipitated and immunoblotted using Myc or/and Flag antibody.
Recent studies have indicated that an NES is located at the N terminus of the IκBα protein, which functions to export the NF-κB from the nucleus to the cytoplasm 22, 23. p28GANKconsists of five ankyrin repeats that are flanked by two tails (Figure 4C), and actively shuttles between the nucleus and cytoplasm (Figure 3A). We constructed various mutants of p28GANK by deleting a tail sequence or each of the ankyrin repeats to identify where the NES is located and which segment is required for the interaction of p28GANK with RelA. As can be seen, only the mutant p28GANK depleted of the N-terminal tail domain (amino acids 1-38) co-immunopreciptated with RelA (Figure 4D), suggesting that all five ankyrin repeats and the C-terminal tail domain (amino acids 39-226) are required for p28GANK binding to RelA. The N-terminal deletion mutant (dN) runs lower on the gel than all other mutants, because dN was not co-fused with HA-tag while others were during their construction.
Analysis of p28GANK structures required for NF-κB nuclear export
In general, IκBα contains leucine-rich NES transporting NF-κB back to the cytoplasm 24, 25. To confirm whether p28GANK contains NES that contributes to its translocation, we transfected the p28GANK mutants and observed their distribution by immunostaining in 293T cells. Only the mutant with the N-terminal tail deleted was present in the nucleus (Figure 5A), indicating that p28GANK contains a putative NES at its N terminus that mediates its shuttling out of the nucleus.
Structural analysis of the p28GANK required for NF-κB nuclear transport. (A) Localization of p28GANK and its deletion mutants in cells. HEK293 cells were transfected with Myc-tagged p28GANK or deletion mutants. Red signals represent the localization of p28GANK and the deletion mutants, and blue signals represent the localization of the nuclei. (B) Localization of RelA is affected by overexpresssion of p28GANK or its depletion mutants. HEK293 cells were co-transfected with Flag-tagged RelA and Myc-tagged IκBα, Myc-tagged p28GANK, or Myc-tagged RelA deletion mutants. Red signals represent the localization of RelA, and blue signals represent the localization of the nuclei.
We next examined the ability of mutant p28GANKproteins to export RelA to the cytoplasm. Overexpressed RelA in 293A cells was predominantly localized in the nucleus. Co-expression with either wild-type p28GANK or IκBα promoted RelA to re-localize in the cytoplasm, but all the mutant p28GANK proteins failed to do so (Figure 5B and data not shown). The failure of p28GANK mutants to retain RelA in the cytoplasm could arise from the removal of NES in mutant p28GANK or from defects in association with RelA. These findings suggest that p28GANK accelerates nuclear export of RelA, which requires the N-terminal domain of p28GANK.
Discussion
In our study, we found that the oncoprotein p28GANK inhibits NF-κB transcriptional and DNA-binding activity. Our data further confirm that p28GANK is a nuclear-cytoplasmic shuttling protein that directly interacts with NF-κB and accelerates NF-κB export from the nucleus.
The prevailing view is that NF-κB proteins, in complex with IκB family molecules, are sequestered in the cytoplasm in an inactive state. After activation by a large number of inducers, the IκB proteins undergo stimuli-coupled phosphorylation, ubiquitylation and proteasome-mediated degradation, which result in the liberation of the NF-κB dimers followed by the nuclear translocation of NF-κB. However, recent studies have indicated that IκBα, IκBβ and IκBε are not essential for the cytoplasmic sequestration of NF-κB and that there are other cellular factors that retain NF-κB in the cytoplasm, which are unresponsive to stimuli 26. In our study, we found that the p28GANK protein associates with NF-κB and retains it in the cytoplasm, even in the absence of IκB proteins (Chen and Li, in submission). Fujita et al. have also found another oncoprotein, hepatoma substracted-cDNA library clone one (HSCO) 27, that binds to NF-κB and accelerates its export from the nucleus independent of IκBα. Together with these data, our observations therefore demonstrate that there are indeed cellular proteins other than the known IκB family molecules that associate with NF-κB and regulate its cytoplasmic retention under basal conditions.
Traditionally it was believed that the inactive NF-κB:IκBα complex continuously shuttles in and out of the nucleus because IκBα masks one of the two nuclear localization signals (NLSs) in an NF-κB dimer and has an NES at its N terminus to achieve a dynamic balance of continuous movement between the nucleus and cytoplasm 28, 29. Here, we found that p28GANKcan also act as a nuclear-cytoplasmic shuttling protein to export NF-κB from the nucleus, and that all ankyrin repeats of p28GANK are indispensable for the interaction with NF-κB (Figures 4D and 5B). We constructed various RelA mutants and p28GANK mutants to determine their interaction mechanisms. Although the NLS of RelA seems not to be involved in the binding, we cannot rule out the possibility of p28GANK masking this domain to maintain NF-κB inactivity. Next, we identified the potential location of the NES of p28GANK as being at its N terminus (amino acids 1-38), which is responsible for the export of both p28GANK itself and the NF-κB:p28GANK complex out of the nucleus. Interestingly, our data showed that although the effects of p28GANK on NF-κB DNA-binding activity and nuclear export are no stronger than that of IκBα, p28GANK inhibits NF-κB transcriptional activity more significantly than IκBα. In addition to the cytoplasmic regulation of NF-κB, post-translational modifications of the NF-κB subunits and the histones that surround the NF-κB target genes have a key role in regulating transcription 30, 31. As additional studies in our laboratory have revealed that p28GANK can directly decrease NF-κB acetylation, it is possible that there are other modifications of NF-κB, caused by p28GANK along with some unknown repressors, that decrease NF-κB transcriptional activity.
Active NF-κB participates in the control of various target genes, including chemokines, immune receptors, adhesion molecules, stress response genes, regulators of apoptosis, transcription factors, growth factors, enzymes and cell-cycle regulators 17, 32. RelA can be thought of as an anti-apoptotic or pro-apoptotic factor depending on the stimuli type and cell background 12, 16, 33, 34, 35. Investigation of the expression of both NF-κB anti-apoptotic and pro-apoptotic target genes will help us to assess how p28GANK affects RelA apoptotic function.
Hepatocellular carcinoma is one of the most common cancers worldwide and develops frequently in the context of chronic hepatitis, characterized by liver inflammation and hepatocyte apoptosis 36, 37. NF-κB is constitutively activated in many tumors and is thought to provide a crucial survival signal that assists cancer cells to escape apoptosis. However, other studies have revealed an anti-tumor function for NF-κB in HCC 38. Partial inhibition of NF-κB led to liver cancer following chemically induced liver damage 39. Overexpression of the oncoprotein p28GANK in HCC acts as a small versatile cell-cycle regulator that regulates the activities of Rb and p53 3, 6. Our study suggests that the regulation of NF-κB by p28GANK might have critical roles in a variety of physiological and pathological process. Further details about the molecular mechanisms underlying NF-κB activity regulated by p28GANK will contribute to an understanding of the regulation of NF-κB pathways, which will provide a platform for developing specific therapeutics for diverse diseases.
References
Baldwin AS . The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol 1996; 14:549–583.
Ghosh S, May MJ, Kopp E . NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 1998; 16:225–260.
Higashitsuji H, Itoh K, Nagao T, et al. Reduced stability of retinoblastoma protein by gankyrin, an oncogenic ankyrin-repeat protein overexpressed in hepatomas. Nat Med 2000; 6:96–99.
Hori T, Kato S, Saeki M, et al. cDNA cloning and functional analysis of p28 (Nas6p) and p40.5 (Nas7p), two novel regulatory subunits of the 26S proteasome. Gene 1998; 216:113–122.
Dawson S, Apcher S, Mee M, et al. Gankyrin is an ankyrin-repeat oncoprotein that interacts with CDK4 kinase and the S6 ATPase of the 26S proteasome. J Biol Chem 2002; 277:10893–10902.
Higashitsuji H, Itoh K, Sakurai T, et al. The oncoprotein gankyrin binds to MDM2/HDM2, enhancing ubiquitylation and degradation of p53. Cancer Cell 2005; 8:75–87.
Li HH, Fu XY, Chen Y, et al. Use of adenovirus-delivered siRNA to target oncoprotein p28GANK in hepatocellular carcinoma. Gastroenterology 2005; 128:2029–2041.
Sen R, Baltimore D . Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 1986; 46:705–716.
Karin M, Ben-Neriah B . Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol 2000; 8:621–663.
Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S . Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev 1995; 9:2723–2735.
Bonizzi G, Karin M . The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol 2004; 25:280–288.
Barkett M, Gilmore TD . Control of apoptosis by Rel/NF-κB transcription factors. Oncogene 1999; 18:6910–6924.
Lin A, Karin M . NF-κB in cancer: a marked target. Semin Cancer Biol 2003; 13:107–114.
Beg AA, Baltimore D . An essential role for NF-κB in preventing TNF-α-induced cell death. Science 1996; 274:782–784.
Kaltschmidt B, Kaltschmidt C, Hofmann TG, Hehner SP, Droge W, Schmitz ML . The pro- or anti-apoptotic function of NF-κB is determined by the nature of the apoptotic stimulus. Eur J Biochem 2000; 267:3828–3835.
Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green DR . DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-κB and AP-1. Mol Cell 1998; 1:543–551.
Pahl HL . Activators and target genes of Rel/NF-κB transcription factors. Oncogene 1999; 18:6853–6866.
Ryan KM, Ernst MK, Rice NR, Vousden KH . Role of NF-κB in p53-mediated programmed cell death. Nature 2000; 404:892–897.
Kudo N, matsumori N, Taoka H, et al. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Pro Natl Acad Sci USA 1999; 96:9112–9117.
Baeuerle PA . IκB-NF-κB structures: at the interface of inflammation control. Cell 1998; 95:729–731.
Huxford T, Huang DB, Malek S, Ghosh G . The crystal structure of the IκB/NF-κB complex reveals mechanisms of NF-κB inactivation. Cell 1998; 95:759–770.
Huang TT, Kudo N, Yoshida M, Miyamoto S . A nuclear export signal in the N-terminal regulatory domain of IκB controls cytoplasmic localization of inactive NF-κB/IκB complexes. Proc Natl Acad Sci USA 2000; 97:1014–1019.
Huang TT, Miyamoto S . Postrepression activation of NF-κB requires the amino-terminal nuclear export signal specific to IkB. Mol Cell Biol 2001; 21:4737–4747.
Arenzana-Seisdedos F, Turpin P, Rodriguez M, Thomas D, Hay RT, Virelizier JL, Dargemont C . Nuclear localization of IκBα promotes active transport of NF-κB from the nucleus to the cytoplasm. J Cell Sci 1997; 110:369–378.
Rodriguez MS, Thompson J, Hay RT, Dargemont C . Nuclear retention of IκBα protects it from signal-induced degradation and inhibits nuclear factor κB transcriptional activation. J Biol Chem 1999; 274:9108–9115.
Vinay T, Ricardo GC, Masahito I, Verma IM . Distinct roles of IκB proteins in regulating constitutive NF-κB activity. Nat Cell Biol 2005; 7:921–923.
Higashitsuji H, Nagao T, Nonoguchi K, Fujii S, Itoh K, Fujita J . A novel protein overexpressed in hepatoma accelerates export of NF-κB from the nucleus and inhibits p53-dependent apoptosis. Cancer Cell 2002; 2:335–346.
Johnson C, Van Antwerp D, Hope TJ . An N-terminal nuclear export signal is required for the nucleocytoplasmic shuttling of IκBα. EMBO J 1999; 18:6682–6693.
Birbach A, Gold P, Binder BR, Hofer E, de Martin R, Schmid JA . Signaling molecules of the NF-kappa B pathway shuttle constitutively between cytoplasm and nucleus. J Biol Chem 2002; 277:10842–10851.
Chen LF, Greene WC . Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol 2004; 5:392–401.
Zhong H, May MJ, Jimi E, Ghosh S . The phosphorylation status of nuclear NF-kappaB determines its association with CBP/p300 or HDAC-1. Mol Cell 2002; 9:625–636.
Aggarwal BB . Nuclear factor-κB: the enemy within. Cancer Cell 2004; 6:203–208.
Perkins ND . The Rel/NF-kappa B family: friend and foe. Trends Biochem Sci 2000; 25:434–440.
Wang SW, Kotamraju S, Konorev E, Kalivendi S, Joseph J, Kalyanaraman B . Activation of nuclear factor-κB during doxorubicin-induced apoptosis in endothelial cells and mocytes is pro-apoptotic: the role of hydrogen peroxide. Biochem J 2002; 367:729–740.
Spalding AC, Jotte RM, Scheinman RI, et al. TRAIL and inhibitors of apoptosis are opposing determinants for NF-κB-dependent, genotoxin-induced apoptosis of cancer cells. Oncogene 2002; 21:260–271.
Motola-Kuba D, Zamora-Valdes D, Uribe M, Mendez-Sanchez N . Hepatocellular carcinoma. An overview. Ann Hepatol 2006; 5:16–24.
Okuda K . Hepatocellular carcinoma. J Hepatol 2000; 32:225–237.
Luedde T, Beraza N, Kotsikoris V, et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell 2007; 11:119–132.
Maeda S, Kamata H, Luo JL, Leffert H, Karin M . IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 2005; 121:977–990.
Acknowledgements
We thank Dr IM Verma (UCSD, USA) and Dr WC Greene (UCSF, USA) for the RelA, p50 and IκBα plasmids. Research was supported by grants from National Natural Science Foundation of China (30530790, 30620130434, 30428006 and 30500275).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Li, H., Fu, J. et al. Oncoprotein p28GANK binds to RelA and retains NF-κB in the cytoplasm through nuclear export. Cell Res 17, 1020–1029 (2007). https://doi.org/10.1038/cr.2007.99
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2007.99
Keywords
This article is cited by
-
Gankyrin modulated non-small cell lung cancer progression via glycolysis metabolism in a YAP1-dependent manner
Cell Death Discovery (2022)
-
Gankyrin as a potential therapeutic target for cancer
Investigational New Drugs (2017)
-
Gankyrin facilitates follicle-stimulating hormone-driven ovarian cancer cell proliferation through the PI3K/AKT/HIF-1α/cyclin D1 pathway
Oncogene (2016)
-
Gankyrin drives malignant transformation of chronic liver damage-mediated fibrosis via the Rac1/JNK pathway
Cell Death & Disease (2015)
-
The nuclear signaling of NF-κB: current knowledge, new insights, and future perspectives
Cell Research (2010)