Abstract
The effect of peripheral nerve (PN)on neurite outgrowth from retinal explants of adult hamsters was examined. Cultures of retinal explants, and co-cultures of retinal explants and PN were performed using chick retinal basement membrane (BM) as substrate. The presence of PN increases the number and length of neurite outgrowth. In addition, a high proportion of neurites situated close to PN tend to grow towards it. Since there was no contact between retinal explants and PN, we suggest that PN might secrete diffusible substances to attract the neurites to grow towards it.
Similar content being viewed by others
Introduction
Recent experiments showed that damaged retinal ganglion cells of adult mammals can regenerate extensively into a segment of peripheral nerve grafted into the eye of rats1 or hamsters2, 3, 4. In addition, the graft in retina seems to play an active role in attracting and/or guiding the regenerating axons to grow into it2. In this work, we co-cultured segments of PN with strips of adult hamster retina to see the effects of PN on the neurite growth from retinal explants. The resuits suggest that PN segments may release into the environment soluble factors which seem to enhance the number and length of neurites outgrowth and affect the direction of growing neurites from adult hamster retinal neurons.
Materials and Methods
In order to select the appropriate substrate for neurite growth from adult hamster retinal neurons, we cultured retinal strips on different substrates in a set of preliminary experiments. The substrates tested included collagen,laminin, poly-L-lysine and basement membrane (BM) from retina of chick embryo of 6- and 12- day old (E6 and E12).The collagen solution was obtained from rat tail as described elsewhere5. A final collagen concentration of 0.4mg/ml was coated onto Petriperm (Heraeus) dishes. Laminin (1 μg or 50 μg per ml; Bethesda Res. Lab.) was coated onto glass cover slips which had been treated with poly-L-lysine (Sigma, MW 52,000, I00 μg/ml).
Each Petriperm dish was incubated with two ml of poly-L-lysine (100 μg/ml) for 30 rain. After drying, the dish was put under UV light for 10 rain and then calcium and magnesium free (CMF) Hank's solution was added into the dish. The Hank's solution should be removed before using the dish for preparation of BM.The BM was obtained from E6 or E12 chick retina6 and Halfter, personal communication). The retina was dissected in CMF Hank's solution. The whole retina from chick was spread out on Sartorius black filter (SM 13006) with photoreceptot cell layer facing the filter (Fig. 1). The filter was then turned upside-down onto a poly-L-lysine coated dish prepared as above. The filter was removed 10 min later with retina still sticking to it while BM was left on the dish.
Culture medium
The culture medium consisted of Eagle's modified minimum essential medium complemented with 2.4% NaHCO3 and 5 mM Hepes, 10% fetal bovine serum, 2% chick serum, 1% chick embryo extract, 2 mM Glutamine, penicillin and streptomycin.
Retinal strip cultures
1. Culturing on poly-L-lysine, laminin and collagen treated dishes.
A whole hamster retina was spread out on a filter. Strips of 600 μm wide were cut using a tissue chopper (Mcllwain). They were then put onto the dishes with different culture substrates with ganglion cell layer facing downward: put up to 10 strips per dish. Culture medium was added and the cultures were placed in an incubator at 37°C with 4% CO2. Unlike the other dishes, the collagen treated dishes, were incubated for 2 hr before adding 2 ml culture medium. In all cases, retinal strips were cultured for 5 days, then fixed with 4% formalin.
2. Culturing of chick basement membranes.
After spreading a hamster retina on a filter, it was cut into 600 μm wide strips (Fig. 1). Two strips were selected and put on E12 BM (prepared as described above). Only one retinal strip was put on E6 BM because of its small size. 2 ml of culture medium was added and the dishes placed in an incubator at 37°C with 4% CO2. Retinal strips were cultured for 3–5 days, then fixed with formalin.
3. Identification of retinal ganglion cells in cultures.
After exposing the anterior portion of the superior colliculus in 3 adult hamsters by surgically removing their overlying cortex, a slit was made in the anterior tip of the superior colliculus and a small piece of gelform (Upjohn) soaked in horseradish peroxidase (HRP; Sigma, type VI, 50% solution in saline) was placed into the slit. The contralateral retina was removed two days later and was cultured on E12 chick BM for 4 days as described above. The retinal explants were then fixed and processed for HRP histochemistry with tetramethyl benzidine (TMB) as described before 1.
Retinal strips and PN cultures
Two retinal strips of 7–8 mm in length obtained from adult hamster were put on E12 chick retinal BM. In addition, a piece of predegenerated PN (3–4 mm long) was put between two retinal strips as in Fig. 2. In order to prevent cell migration from PN, it was put inside a glass tube (0.9 mm inner diameter, open at both ends). For any given culture, the retina and PN should obtained from the same animal. Sciatic nerve, which was used as PN source, was crushed 4–10 days prior to culture experiment. The proximal end of the crushed nerve was tied with suture and the nerve segments were removed just before use.
A schematic diagram showing two strips of adult hamster retinas(R) co-cultured with a segment of peripheral nerve (PN) situated inside a glass tube (GT). BM from an E12 chick embryo is used as substrate (E12 BM). Numerals 1, 2. 3, and 4 on retinal strips denote zones of 1 mm each, zone 1 starts from the mouth of glass tube. B, back-to-PN sides; F, face-to-PN sides.
After adding 2 ml culture medium, the cultures were placed in an incubator at 37°C with 4%o CO2. The retinal strips were cultured for 5 days with no disturbance of dishes. Fixed with 4%o formalin.
Analysis of cultures
1. Retinal strip cultures.
After fixation, cultures were examined with an inverted microscope (Zeiss IM 35)and phase contrast optics. Ten longest neurites were measured from each retinal strip and their average length was used as an indicator of growth potential of each retinal strip cultured under different conditions.
2. Retinal strips and PN cultures.
The average length of neurite growth was obtained for each retinal strip as described above. In addition, the growth pattern of all neurites situated between the open end of glass tube and 4 mm of the retinal strips was analysed (Fig. 2). The 4 mm segment of retina was divided into 4 zones (Fig. 2, 3).
Histograms demonstrating the percent of three neurite growth pattern of each zone in retinal strip co-cultured with PN. The experimental paradigm is illustrated in Fig. 2.
A, Neurite growth pattern on F side: B,Neurite growth pattern on B side. Black bars. positive curved neurites, strap bars. straight neurites. Open bars, negative curved neurites. Further details, see Tables 1 and 2.
In order to quantify the effect of PN on growth pattern of neurites, the following method was designed to define their growth pattern. The exit point of a neurite from retinal strip was noted. Then, its growing tip was located and a perpendicular line was drawn from the tip of the neurite to intersect retinal strip at another point. If the distance between these two points was less than 250 μm, the neurite was defined to grow in a straight mode. If this distance was more than 250 μm and the neurite was curving towards glass tube, it was defined as growing towards PN (positive curved neurite). If this distance was more than 250 μm and the neurite was curving away from glass tube, it was defined as growing away from PN (negative curved neurite). The data from each mm zone of retinal strip (on both sides of the strips, Fig. 2) were summed up in Tables 1, and 2. The percentage of positive and negative curved and straight neurites, was calculated for each mm zone (Table 1, 2, and Fig. 3).
Results
Effect of different substrates on neurite outgrowth foe retinal explants
Number of neurite growing out from adult retinal explants was small, irrespective of the substrate used. However, length of neurite growth from adult hamster retina varied according to the substrate used (Fig. 4). An average neurite outgrowth length of 155 μm was obtained when poly-L-lysine was used as substrate. The situation was similar for collagen which demonstrated an averageneurite outgrowth length of 188 μm. Neurites grew markedly better on laminin and reached an average outgrowth length of 582 μm. However, neurite seemed to grow even better on retinal BM from E6 and E12 chicken. With average outgrowth length of 690 and 823 μm respectively.
Histogram showing the length of neurite from retinal strips growing on different substrates. Shaded areas represent average neurite length obtained from all the experiments using a particular substrate. Enclosed white areas denote length of the longest neurite growing on each substrate.
P, poly-L-lysine; C, collagen;
L, laminin;
E6 and El2, BM from E6 and El2 chick embryos;
El2 + PN, BM from El2 chick embryo with PN in culture.
The origin of neurites in vitro
After 4 days in cultures, many HRP labelled cell bodies were observed in the retinal strips obtained from animals in which HRP had been administered to the superior colliculus. These cell bodies were found in retinal ganglion cell layer. More importantly,HRP reaction products could be seen in the initial portion of some outgrowth neurites (not shown).
Effect of PN on neurite outgrowth
Since E12 BM seemed to be the best substrate for neurite outgrowth from adult retinal explants, it was used for experiments on the effects of PN on neurite growth in cultures.
Length of neurite outgrowth.
There seems to be difference in the length of neurite outgrowth in retinal strip culture with or without PN after 5 days in cultures (Fig. 4). The average length of neurite in retinal strip cultures alone was 823 μm whereas in retinal explants and PN co-cultures, the average neurite length was 1305 μm. This increase of 58.6% suggests that the presence of PN enhanced the growth potential of neurites.
Number of neurites.
In retinal explants and PN co-cultures, neurite number in different zones of retinal strip increased gradually as they approached the opening of glass tube (Table 1 and 2). This was true on both sides of the retinal strip suggesting an enhancement effect of PN on the number of growing neurites
Direction of neurite outgrowth.
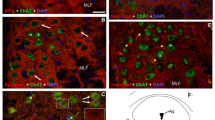
40 retinal strips were cultured on 20 E12 BM. 416 neurites on the F side and 329 on the B side were classified as being straight, positive or negative curved neurites as defined in Materials and Methods' section. Results indicated that the percentage of positive curved neurites was substantially higher in zone 1 on F side, but not on B side (Table 1 and 2, Fig. 4). Two neurites growing towards the glass tubes (positive curved neurites) are illustrated in Fig. 5. Out of 187 axons analysed in zone 1 on experimental F side, 117 of them curved towards PN, 48 were straight, and only 22 curved away from PN (Table 1).There was no obvious difference in the direction of neurite growth on B side, or in the remaining zones on F side. The maximum distance between PN and neurites as they emerged from zone 1 on F side, was about 2.5–2.8mm, thus 2.8 mm seems to be the limiting distance beyond which the direction of neurite growth was not affected by PN. No neurite has been shown to grow into the glass tube and to contact PN.
In most cultures, no cell was observed to migrate from PN, via glass tube, onto BM. But a few cells were seen in a few cultures. However, these cells were situated immediately adjacent to the mouth of glass tube and they did not in contact with any neurites.
Discussion
Neurite outgrowth was observed on various substrates, although there were differences in growth potential of neurites growing on different substrates. Neurite outgrowth on collagen was slightly better than that on poly-L-lysine, but laminin turned out to be a much better substrate than collagen or poly-L-lysine. The fact that laminin is an effective substrate for enhancing neurite outgrowth from retina has been shown in retinal cultures from fetal mice7 or adult rat8. Lamininis an important component in BM9 and its presence was demonstrated on E6 and E12 (Liu and So, unpublished results) and on E5 to E86 chick retinal BM with immunocytochemical method. This might explain why chick retinal BM was best substrate for neurite outgrowth from adult hamster retinas. That BM is a better substrate than laminin might be due to the fact that in addition to laminin, other neurite promoting substances such as heparan sulfate, proteoglycan might present in chick retinal BM. These neurite promoting substances on BM, in contrast to laminin which was plated on a culture dish, are more likely to be in their normal biological configuration, thus providing a more favorable environment for neurite growth.
Our results suggest that the growth pattern of neurites from adult hamster retinal explants can be modified by soluble factors secreted by the segments of predegenerated sciatic nerve cultured in the same dish. The presence of PN seems also increase the length and number of neurite outgrowth, however, the effect decreases with their distance from PN. Thus, more outgrowing neurites were observed in areas (of retinal explant) close to PN. In addition, the neurites within about 2.8 mm from PN were attracted to grow towards the PN while those situated outside this zone did not seem to be affected.
Although we conclude that PN secretes diffusible substances in vitro to attract regenerating axons, we have to consider the possibility that in in vivo situation Schwann or other cell types might migrate out of PN onto retina to guide damaged axons to grow towards the nerve. In fact, in a couple of experiments, in which retinal strip were co-cultured with PN which was not put into a glass tube, we could observe a lot of cells migrating out of the nerve to intermingle with neurites growing out from retinal explants. Nevertheless. since no contact between neurites and cellular components from PN was observed in our experiments with the nerve cultured inside a glass tube, we suggest that the diffusible substances secreted by PN seem to be sufficient to alter the growth of neurites from adult hamster retinal neurons.
Various cellular components in injured PN could be sources of trophic factors. Schwann cells are likely to be an important contributor since they multiply after PN is injured10, and it has been shown recently that conditioned medium from Schwann cells in culture seems to stimulate neurite outgrowth11. Other trophic factors have also been shown to be released from PN in in vitro experiments 12, 13. These factors seem to enhance neurite outgrowth of dorsal root ganglion neurons from embryonic rats 13. In addition, Varon and his colleagues have also illustrated neurite-promoting activity in fluid collected from chambers surrounding regenerating rat sciatic nerve14. Results from our experiments suggest that the trophic factors released from segments of PNs are also able to enhance the number and length and to influence the direction of neurite outgrowth from retinal explants of adult hamsters. Although we did not positively identify the source of all these neurites, we suppose that they are from retinal ganglion cells. Since the retinal ganglion cell layer of retinal explants in culture was in direct contact with substrate, the axotomized retinal ganglion cells, in comparison to the other retinal neurons, should have a much higher chance to grow neurites on the substrate. Indeed, by applying HRP to the superior colliculus in 3 animals, we have been able to show that many retinal ganglion cells survive in our culture system and HRP reaction products have also been observed in initial portion in some of the growing neurites. It has been shown from other peripheral nerve grafting experiments that diffusible factors have a'rescue' effect in the retinal ganglion cell layer15 and reduce the effects of axotomy on damaged retinal ganglion cells16 in adult rats. We do not know what these trophic factors are at the moment. They can be nerve growth factor-like substances or other growth related substances12, 13, 17, 18, 19, 20. Of particular interest is the recent work showing that fibroblast growth factors (FGF) seems to promote the survival of axotomized retinal ganglion cells of adult rat21. Thus, it will be of interest to determine if FGF is also being released by injured peripheral nerve.
References
So KF, Aguayo AJ . Lengthy regrowth of cut axons from ganglion cells after peripheral nerve transplantation into the retina of adult rat. Brain Research 1985; 328: 349–354.
So KF, Xiao YM, Diao YC . Effects on the growth of damaged ganglion cell axons after peripheral nerve transplantation in adult hamster. Brain Research 1986; 377: 168–172.
Xiao YM, So KF . Regeneration of ganglion cell axons after peripheral nerve transplantation into the retina of adult golden hamsters. Acta Anatomica Sinica 1987 ;18 141–146.
Cho EYP, So KF . Rate of regrowth of damaged retinal ganglion cell axons regenerating in a peripheral nerve graft in adult hamsters. Brain Research 1987; 419: 369–374.
Halfter W, Newgreen DF, Sauter J, Schwarz U . Oriented axon outgrowth from avian embryonic retinae in culture. Dev Biol 1983; 95: 56–64.
Halfter W, Reckhaus W, Kroger S . Nondirected axonal growth on basal lamina from avian embryonic neural retina. J Neurosci 1987; 7: 3712–3722.
Smalheiser NR, Crain SM, Reid LM . Laminin as a substrate for retinal axons in Vitro. Dev Brain Research 1984; 12: 136–140.
Ford-Halevinski TS, Hopkins JM, McCoy JP, Agranoff BW . Laminin supports neurite outgrowth from explants of axotomized adult rat retinal neurons. Dev Brain Research 1986; 28: 121–126.
Timpl R, Rohde H . Laminin-a glycoprotein from basement membranes. J Biol Chem 1979 ; 254: 9933–9937.
Aguayo A J, Epps J, Charron L, Bray GM . Multipotentiality of Schwann Cells in crossanastomosed and grafted myelinated and unmyelinated nerves: quantitative microscopy and radioautography. Brain Research 1976; 104: 1–20.
Assouline JG, Bosch P, Lim R, Kim IS, Jensen R, Pantazis NJ . Rat astrocytes and Schwann cells in culture synthesize nerve growth factor-like neurite-promoting factors. Dev Brain Research 1987; 31: 103–118.
Richardson PM, Ebendal T . Nerve growth activities in rat peripheral nerve. Brain Research 1982; 246: 57–64.
Windebank AJ, Poduslo JF . Neuronal growth factors produced by adult peripheral nerve after injury. Brain Research 1986; 385: 197–200.
Longo FM, Hayman EG, Davis GE, Ruoslahti E, Engvall E, Manthorpe M, Varon S . Neurite-promoting factors and extracellular matrix components accumulating in vitro within nerve regenerating chambers. Brain Research 1984; 309: 105–117.
Turner JE, Blair JR, Chappel ET . Peripheral nerve implant effects on survival of retinal layer cells after axotomy initiated by penetrating lesion. Brain Research 1987 ;419: 46–54.
Villegas-Perez MP, Vida-Sanz M, Aguayo AJ . Effects of axotomy and PN grafting on adult rat retinal ganglion cells. Soc Neurosci Abstr 1986; 12: 700.
Cotman CW, Nieto-Sampedro M . Cell biology of synaptic plasticity. Science 1984;225: 1287–1294.
Skene PJH, Shooter EM . Denervated sheath ceils secrete a new protein after nerve injury. Proc Natl Acad Sci USA 1983; 80: 4169–4173.
Thoenen H, Edgar D . Neurotrophic factors. Science 1985; 229: 238–242.
Varon S, Manthorpe M, Longo FM, Williams LR . Growth factors in regeneration of neural tissues. In: Sell F J, ed. Nerve, organ and tissue regeneration: Research Perspectives. New York: Academia Press, 1983: 127–155.
Sievers J, Hausmann B, Unsicker K, Berry M . Fibroblast growth factors promote the survival of adult rat retinal ganglion cells after transection of the optic nerve. Neurosci Lett 1987; 76: 157–162.
Acknowledgements
We would like to thank Drs. A. Harvey and L.S. Jen for their comments on the manuscript. The assistance of L.Z. Jiang (tissue culture), K.C. Lau (histology), S.Fung (typing), K.C. Kung and J. Leung (photography) are greatly appreciated. L. Liu is a Beijing-Hong Kong Academic Exchange Scholar on leave from Shanghai Institute of Cell Biology, Academic Sinica, Shanghai, China. The research is supported by grants from the University of Hong Kong and Croucher Foundation.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liu, L., So, K. Effect of peripheral nerve on the neurite growth from retinal explants in culture. Cell Res 1, 77–87 (1990). https://doi.org/10.1038/cr.1990.8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/cr.1990.8