Abstract
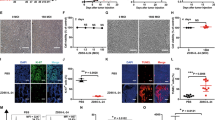
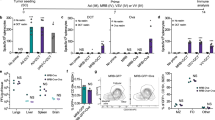
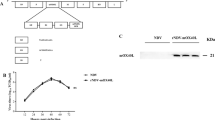
Inactivated Sendai virus particles (hemagglutinating virus of Japan envelope; HVJ-E) are considered to be safe and efficient non-viral vectors used for drug delivery, since they can incorporate DNA, RNA, proteins and drugs. We have recently found that HVJ-E has a novel antitumor immune effect using a colon cancer model. HVJ-E has also been shown to have both direct and immune-mediated indirect actions against malignancy. Intratumoral injection of an inactivated HVJ-E solution significantly reduced the tumor volume and prevented spontaneous lung metastasis, leading to an increased overall survival in C57/BL6 mice transplanted with B16/BL6 mouse melanoma cells, and even in immunodeficient mice transplanted with Mewo human melanoma cells. No severe adverse effects including laboratory data abnormalities or anaphylactic reactions were observed. The comprehensive mechanism(s) underlying the immunological effects of HVJ-E appear to include not only enhanced effector T cell- and/or natural killer (NK) cell-mediated immunity, but also rescue from regulatory T cell (Treg)-mediated immunosuppression, presumably through the interleukin-6 secretion from dendritic cells stimulated by HVJ-E. Since a protocol for a clinical study of HVJ-E in malignant melanoma was approved in 2009 by the ethics committee of Osaka University and of the Medical Center for Translational Research in Osaka University Hospital, a phase I/IIa study for advanced malignant melanoma patients was just started. In this review, we show several favorable results regarding the antitumor effects of HVJ-E and describe the novel mechanism underlying this tumor immune response. Since we are conducting a phase I/IIa clinical trial using HVJ-E in advanced melanoma patients on the basis of preclinical results, detailed clinical information and immune-monitoring data are also introduced. The development of new therapeutic modalities for advanced melanoma patients is urgently needed, and we hope that HVJ-E may provide one such treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Abbreviations
- CTL:
-
cytotoxic T lymphocyte
- CTLA-4:
-
cytotoxic T-lymphocyte antigen 4
- HVJ-E:
-
hemagglutinating virus of Japan envelope
- PD-1:
-
programmed cell death-1
- Treg:
-
regulatory T cell
References
Lens MB, Eisen TG . Systemic chemotherapy in the treatment of malignant melanoma. Expert Opin Pharmacother 2003; 4: 2205–2211.
Li Y, McClay EF . Systemic chemotherapy for the treatment of metastatic melanoma. Semin Oncol 2002; 29: 413–426.
Ridolfi R, Petrini M, Fiammenghi L, Stefanelli M, Ridolfi L, Ballardini M et al. Improved overall survival in dendritic cell vaccination-induced immunoreactive subgroup of advanced melanoma patients. J Transl Med 2006; 4: 36.
Wei Y, Sticca RP, Holmes LM, Burgin KE, Li J, Williamson J et al. Dendritoma vaccination combined with low dose interleukin-2 in metastatic melanoma patients induced immunological and clinical responses. Int J Oncol 2006; 28: 585–593.
Goto S, Kaneko T, Miyamoto Y, Eriguchi M, Kato A, Akeyama T et al. Combined immunocell therapy using activated lymphocytes and monocyte-derived dendritic cells for malignant melanoma. Anticancer Res 2005; 25: 3741–3746.
Homma S, Kikuchi T, Ishiji N, Ochiai K, Takeyama H, Saotome H et al. Cancer immunotherapy by fusions of dendritic and tumor cells and rh-IL-12. Eur J Clin Invest 2005; 35: 279–286.
Rosenberg SA, Zhai Y, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM et al. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J Natl Cancer Inst 1998; 90: 1894–1900.
Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Topalian SL, Sherry RM et al. Recombinant fowlpox viruses encoding the anchor-modified gp100 melanoma antigen can generate antitumor immune responses in patients with metastatic melanoma. Clin Cancer Res 2003; 9: 2973–2980.
Rosenberg SA . Cell transfer immunotherapy for metastatic solid cancer—what clinicians need to know. Nat Rev Clin Oncol 2011; 8: 577–585.
Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008; 26: 5233–5239.
Hodi FS, O’day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 368: 711–723.
Zitvogel L, Kroemer G . Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology 2012; 1: 1223–1225.
Kaneda Y, Saeki Y, Morishita R . Gene therapy using HVJ-liposomes: the best of both worlds? Mol Med Today 1999; 5: 298–303.
Kaneda Y . A non-replicating oncolytic vector as a novel therapeutic tool against cancer. BMB Rep 2010; 43: 773–780.
Kaneda Y . Virosome: a novel vector to enable multi-modal strategies for cancer therapy. Adv Drug Deliv Rev 2012; 64: 730–738.
Kurooka M, Kaneda Y . Inactivated Sendai virus particles eradicate tumors by inducing immune responses through blocking regulatory T cells. Cancer Res 2007; 67: 227–236.
Okada Y . Sendai virus-induced cell fusion. Methods Enzymol 1993; 221: 18–41.
Kaneda Y . Applications of hemagglutinating virus of Japan in therapeutic delivery systems. Expert Opin Drug Deliv 2008; 5: 221–233.
Kohler G, Milstein C . Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975; 256: 495–497.
Jami J, Grandchamp S . Karyological properties of human-mouse somatic hybrids. Proc Natl Acad Sci USA 1971; 68: 3097–3101.
Kaneda Y, Yamamoto S, Nakajima T . Development of HVJ envelope vector and its application to gene therapy. Adv Genet 2005; 53: 307–332.
Kaneda Y, Nakajima T, Nishikawa T, Yamamoto S, Ikegami H, Suzuki N et al. Hemagglutinating virus of Japan (HVJ) envelope vector as a versatile gene delivery system. Mol Ther 2002; 6: 219–226.
Fujiwara A, Kurooka M, Miki T, Kaneda Y . Intratumoral injection of inactivated Sendai virus particles elicits strong antitumor activity by enhancing local CXCL10 expression and systemic NK cell activation. Cancer Immunol Immunother 2008; 57: 73–84.
Powrie F, Maloy KJ . Regulating the regulators. Science 2003; 299: 1030–1031.
Fehervari Z, Sakaguchi S . Control of Foxp3+CD25+CD4+ regulatory cell activation and function by dendritic cells. Int Immunol 2004; 16: 1769–1780.
Wan S, Xia C, Morel L . IL-6 produced by dendritic cells from lupus-prone mice inhibits CD4+CD25+ T cell regulatory functions. J Immunol 2007; 178: 271–279.
Overwijk WW, Restifo NP . B16 as a mouse model for human melanoma. Curr Protoc Immunol 2001 Chapter 20: Unit 20.1.
Ruiter DJ, Bergman W, Welvaart K, Scheffer E, van Vloten WA, Russo C et al. Immunohistochemical analysis of malignant melanomas and nevocellular nevi with monoclonal antibodies to distinct monomorphic determinants of HLA antigens. Cancer Res 1984; 44: 3930–3935.
Terme M, Ullrich E, Aymeric L, Meinhardt K, Coudert J, Desbois M et al. Cancer-induced immunosuppression: IL-18-elicited immunoablative NK cells. Cancer Res 2012; 72: 2757–2767.
Kaneda Y, Yamamoto S, Nakajima T . Development of HVJ envelope vector and its application to gene therapy. Adv Genet 2005; 53: 303–332.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247.
Acknowledgements
This study was supported by a Grant-in-Aid for Young Scientists (B) (23791268) from the Japanese Ministry of Education, Science, Sports, and Culture and a Grant-in-Aid from the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tanemura, A., Kiyohara, E., Katayama, I. et al. Recent advances and developments in the antitumor effect of the HVJ envelope vector on malignant melanoma: from the bench to clinical application. Cancer Gene Ther 20, 599–605 (2013). https://doi.org/10.1038/cgt.2013.61
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2013.61
Keywords
This article is cited by
-
Intratumoral injection of hemagglutinating virus of Japan-envelope vector yielded an antitumor effect for advanced melanoma: a phase I/IIa clinical study
Cancer Immunology, Immunotherapy (2020)
-
Oncolysis by paramyxoviruses: preclinical and clinical studies
Molecular Therapy - Oncolytics (2015)