Abstract
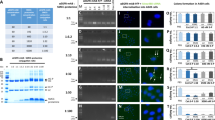
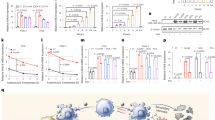
The two membrane-bound proteins, focal adhesion kinase (FAK) and CD44, are involved in processes critical to cancer progression. FAK has an active role in angiogenesis, cell proliferation and cell apoptosis, whereas the heavily glycosylated CD44 has been implicated in cancer metastasis. Here, using short hairpin RNA (shRNA) against FAK and CD44, we demonstrate that simultaneous knockdown of both these genes inhibits cancer growth more efficiently than knockdown of either gene individually. Plasmids targeting these genes or non-relative control sequences were constructed and delivered to ovarian cancer targets by biodegradable poly D,L-lactide-co-glycolide acid nanoparticles (PLGANPs). Nude mice were utilized in an intraperitoneal model of ovarian carcinomatosis to assess antitumor efficacy in vivo. Single gene knockdown resulted in significantly smaller tumors than those observed in the empty-vector control (P’s<0.001). More importantly, knockdown of both genes resulted in tumors smaller than both the empty-vector group (P<0.0001) and the single gene knockdown groups (P’s<0.001). Knockdown of both FAK and CD44 resulted in tumors with inhibited angiogenesis, reduced proliferation and increased apoptosis as compared with controls (P’s<0.001) and single knockdown groups (P’s<0.05). These results indicate that dual knockdown of FAK and CD44 in the tumors of patients with ovarian cancer may have an enhanced therapeutic effect, and point toward a mechanism involving the inhibition of angiogenesis, cellular proliferation and the induction of apoptosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A . Cancer statistics, 2012. CA Cancer J Clin 2012; 62: 10–29.
Davidson B . Anatomic site-related expression of cancer-associated molecules in ovarian carcinoma. Curr Cancer Drug Targets 2007; 7: 109–120.
Sood AK, Coffin JE, Schneider GB, Fletcher MS, DeYoung BR, Gruman LM et al. Biological significance of focal adhesion kinase in ovarian cancer. Am J Pathol 2004; 165: 1087–1095.
Haskell H, Natarajan M, Hecker TP, Ding Q, Stewart J, Grammer JR et al. Focal adhesion kinase is expressed in the angiogenic blood vessels of malignant astrocytic tumors in vivo and promotes capillary tube formation of brain microvascular endothelial cells. Clin Cancer Res 2003; 9: 2157–2165.
Shen TL, Park AYJ, Alcaraz A, Peng X, Jang I, Koni P et al. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J Cell Biol 2005; 169: 941–952.
Halder J, Lin YG, Merritt WM, Spannuth WA, Nick AM, Honda T et al. Therapeutic efficacy of a novel focal adhesion kinase inhibitor TAE226 in ovarian carcinoma. Cancer Res 2007; 67: 10976–10983.
Judson PL, He XP, Cance WG, Le LV . Overexpression of focal adhesion kinase, a protein tyrosine kinase, in ovarian carcinoma. Cancer 1999; 86: 1551–1556.
Halder J, Kamat AA, Landen CN, Han LY, Lutgendorf SK, Lin YG et al. Focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy. Clin Cancer Res 2006; 12: 4916–4924.
Lesley J, Hyman R, Kincade PW . CD44 and its interaction with extracellular matrix. Adv Immunol 1993; 54: 271–335.
Ponta H, Wainwright D, Herrlich P . The CD44 protein family. Int J Biochem Cell Biol 1998; 30: 299–305.
Sillanpää S, Anttila MA, Voutilainen K, Tammi RH, Tammi MI, Saarikoski SV et al. CD44 expression indicated favorable prognosis in epithelial ovarian cancer. Clin Cancer Res 2003; 9: 5318–5324.
Bapat SA, Mali AM, Koppikar CB, Kurrey NK . Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res 2005; 65: 3025–3029.
Li CZ, Liu B, Wen ZQ, Wang CX, Li HY . Inhibition of CD44 expression by small interfering RNA to suppress the growth and metastasis of ovarian cancer cells in vitro and in vivo. Folia Biol (Praha) 2008; 54: 180–186.
Pai SI, Lin YY . Prospects of RNA interference therapy for cancer. Gene Ther 2006; 13: 464–477.
Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V . Rapid endolysosomal escape of poly (d,l-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J 2002; 16: 1217–1226.
Cui FY, Song XR, Li ZY, Li SZ, Mu B, Mao YQ et al. The pigment epithelial-derived factor gene loaded in PLGA nanoparticles for therapy of colon carcinoma. Oncol Rep 2010; 24: 661–668.
Sun C, Yi T, Li S, Qi X, Chen X, Lin H et al. Efficient inhibition of ovarian cancer by short hairpin RNA targeting claudin-3. Oncol Rep 2011; 26: 193–200.
Roscic-Mrkic B, Fischer M, Leemann C, Manrique A, Gordon CJ, Moore JP et al. RANTES (CCL5) uses the proteoglycan CD44 as an auxiliary receptor to mediate cellular activation signals and HIV-1 enhancement. Blood 2003; 102: 1169–1177.
Song XR, Zhao Y, Wu WB, Bi YQ, Cai Z, Chen QH et al. PLGA nanoparticles simultaneously loaded with vincristine sulfate and verapamil hydrochloride: systematic study of particle size and drug entrapment efficiency. Int J Pharm 2008; 350: 320–329.
Song XR, Zhao Y, Hou SX, Xu FY, Zhao RL, He JY et al. Dual agents loaded PLGANPs: systematic study of particle size and drug entrapment efficiency. Eur J Pharm Biopharm 2008; 69: 445–453.
Lin XJ, Chen XC, Wei YQ, Zhao J, Fan L, Wen Y et al. Efficient inhibition of intraperitoneal human ovarian cancer growth and prolonged survival by gene transfer of vesicular stomatitis virus matrix protein in nude mice. Gynecol Oncol 2007; 104: 540–546.
Song XR, Cai Z, Zheng Y, He G, Cui FY, Gong DQ et al. Reversion of multidrug resistance by co-encapsulation of vincristine and verapamil in PLGA nanoparticles. Eur J Pharm Sci 2009; 37: 300–305.
Brown TA, Bouchard T, John TS, Wayner E, Carter WG . Human keratinocytes express a new CD44 core protein (CD44E) as a heparan-sulfate intrinsic membrane proteoglycan with additional exons. J Cell Biol 1991; 113: 207–221.
Hannon GJ, Rossi JJ . Unlocking the potential of the human genome with RNA interference. Nature 2004; 431: 371–378.
Zhang X, Deng HX, Zhao X, Su D, Chen XC, Chen LJ et al. RNA interference-mediated silencing of the phosphatidylinositol 3-kinase catalytic subunit attenuates growth of human ovarian cancer cells in vitro and in vivo. Oncology 2009; 77: 22–32.
Su D, Deng H, Zhao X, Zhang X, Chen L, Chen X et al. Targeting CD24 for treatment of ovarian cancer by short hairpin RNA. Cytotherapy 2009; 11: 642–652.
Shahzad MM, Lu C, Lee JW, Stone RL, Mitra R, Mangala LS et al. Dual targeting of EphA2 and FAK in ovarian carcinoma. Cancer Biol Ther 2009; 8: 1027–1034.
Canel M, Serrels A, Miller D, Timpson P, Serrels B, Frame MC et al. Quantitative in vivo imaging of the effects of inhibiting integrin signaling via Src and FAK on cancer cell movement: effects on E-cadherin dynamics. Cancer Res 2010; 70: 9413–9422.
Zhao J, Guan JL . Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev 2009; 28: 35–49.
Grisaru-Granovsky S, Salah Z, Maoz M, Pruss D, Beller U, Bar-Shavit R et al. Differential expression of Protease activated receptor 1 (Par1) and pY397 FAK in benign and malignant human ovarian tissue samples. Int J Cancer 2004; 113: 372–378.
Halder J, Landen CN, Lutgendorf SK, Li Y, Jennings NB, Fan D et al. Focal adhesion kinase silencing augments docetaxel-mediated apoptosis in ovarian cancer cells. Clin Cancer Res 2005; 11: 8829–8836.
Skubitz AP . Adhesion molecules. Cancer Treat Res 2002; 107: 305–329.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF . Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003; 100: 3983–3988.
Misra S, Hascall VC, Giovanni CD, Markwald RR, Ghatak S . Delivery of CD44 shRNA/nanoparticles within cancer cells. J Biol Chem 2009; 284: 12432–12446.
Guo Y, Guo H, Zhang L, Xie H, Zhao X, Wang F et al. Genomic analysis of anti-Hepatitis B Virus (HBV) activity by small interfering RNA and lamivudine in stable HBV-producing cells. J Virol 2005; 79: 14392–14403.
Young LS, Mautner V . The promise and potential hazards of adenovirus gene therapy. Gut 2001; 48: 733–736.
Gao Y, Xu Z, Chen S, Gu W, Chen L, Li Y . Arginine-chitosan/DNA self-assemble nanoparticles for gene delivery: in vitro characteristics and transfection efficiency. Int J Pharm 2008; 359: 241–246.
Suzuki R, Namai E, Oda Y, Nishiie N, Otake S, Koshima R et al. Cancer gene therapy by IL-12 gene delivery using liposomal bubbles and tumoral ultrasound exposure. J Control Release 2010; 142: 245–250.
Park IK, Singha K, Arote RB, Choi YJ, Kim WJ, Cho CS . pH-responsive polymers as gene carriers. Macromol Rapid Commun 2010; 31: 1122–1133.
Gou M, Men K, Zhang J, Li Y, Song J, Luo S et al. Efficient inhibition of C-26 colon carcinoma by VSVMP gene delivered by biodegradable cationic nanogel derived from polyethyleneimine. ACS Nano 2010; 4: 5573–5584.
Kang BK, Chon SK, Kim SH, Jeong SY, Kim MS, Cho SH et al. Controlled release of paclitaxel from microemulsion containing PLGA and evaluation of anti-tumor activity in vitro and in vivo. Int J Pharm 2004; 286: 147–156.
Kang SW, Lim HW, Seo SW, Jeon O, Lee M, Kim BS . Nanosphere-mediated delivery of vascular endothelial growth factor gene for therapeutic angiogenesis in mouse ischemic limbs. Biomaterials 2008; 29: 1109–1117.
Qi X, Song X, Liu P, Yi T, Li S, Xie C et al. Antitumor effects of PLGA nanoparticles encapsulating the human PNAS-4 gene combined with cisplatin in ovarian cancer. Oncol Rep 2011; 26: 703–710.
Acknowledgements
This work was kindly supported by grants from the National 973 Basic Research Programs of China (2011CB910703 and 2004CB518800) and Natural Science Foundation (0040215401068).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zou, L., Song, X., Yi, T. et al. Administration of PLGA nanoparticles carrying shRNA against focal adhesion kinase and CD44 results in enhanced antitumor effects against ovarian cancer. Cancer Gene Ther 20, 242–250 (2013). https://doi.org/10.1038/cgt.2013.12
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2013.12
Keywords
This article is cited by
-
The biology and role of CD44 in cancer progression: therapeutic implications
Journal of Hematology & Oncology (2018)
-
Shape dependent cytotoxicity of PLGA-PEG nanoparticles on human cells
Scientific Reports (2017)
-
Efficacy of Piroxicam Plus Cisplatin-Loaded PLGA Nanoparticles in Inducing Apoptosis in Mesothelioma Cells
Pharmaceutical Research (2015)