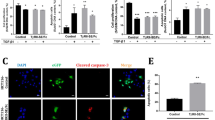
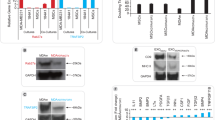
Abstract
Cancer metastasis contributes significantly to cancer mortality and is facilitated by lymphangiogenesis and angiogenesis. Vascular endothelial growth factor-C (VEGF-C) and VEGF-A are involved in lymphangiogenesis and angiogenesis. To inhibit metastasis, combination therapy with vector-based small interfering RNA (siRNA) against VEGF-C and/or VEGF-A was conducted on murine metastatic mammary cancer. Syngeneic, inoculated, metastatic mammary cancers received direct intratumoral injection of plasmid siRNA vector targeting VEGF-C (psiRNA-VEGF-C), VEGF-A (psiRNA-VEGF-A), both VEGF-C and VEGF-A (both psiRNA-VEGF-C and psiRNA-VEGF-A vectors injected, referred to as the psiRNA-VEGF-C+A group) or a scrambled sequence (psiRNA-SCR) as control, once a week for 8 weeks. Gene electrotransfer was performed on the tumors after each injection. Tumor volume was significantly lower in the psiRNA-VEGF-A and the psiRNA-VEGF-C+A groups throughout the study. Lymph node metastasis was significantly less frequent in all therapeutic groups, whereas the multiplicity of lung metastases was significantly lower in the psiRNA-VEGF-C+A group only. All siRNA therapeutic groups showed a significant reduction in the number of dilated lymphatic vessels containing intraluminal cancer cells and microvessel density. Our data suggest that specific silencing of the VEGF-C or VEGF-A gene alone can inhibit lymph node metastasis. However, combination siRNA therapy targeting both VEGF-C and VEGF-A inhibits both lymph node and lung metastasis, rendering this combined therapy more beneficial than either alone. The observed anti-metastatic activity of siRNA-expressing vectors targeting VEGF-C or VEGF-A may be of high clinical significance in the treatment of metastatic breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Abbreviations
- RT-PCR:
-
reverse transcription-PCR
- shRNA:
-
short hairpin RNAs
- siRNA:
-
short interfering RNA
- VEGF:
-
vascular endothelial growth factor
References
Cody III HS, Borgen PI, Tan LK . Redefining prognosis in node-negative breast cancer: can sentinel lymph node biopsy raise the threshold for systemic adjuvant therapy? Ann Surg Oncol 2004; 11 (3 Suppl): 227S–230S.
Joory KD, Levick JR, Mortimer PS, Bates DO . Vascular endothelial growth factor-C (VEGF-C) expression in normal human tissues. Lymphat Res Biol 2006; 4: 73–82.
Salven P, Lymboussaki A, Heikkila P, Jääskela-Saari H, Enholm B, Aase K et al. Vascular endothelial growth factors VEGF-B and VEGF-C are expressed in human tumors. Am J Pathol 1998; 153: 103–108.
Mylona E, Alexandrou P, Mpakali A, Giannopoulou I, Liapis G, Markaki S et al. Clinicopathological and prognostic significance of vascular endothelial growth factors (VEGF)-C and -D and VEGF receptor 3 in invasive breast carcinoma. Eur J Surg Oncol 2007; 33: 294–300.
Nakamura Y, Yasuoka H, Tsujimoto M, Imabun S, Nakahara M, Nakao K et al. Lymph vessel density correlates with nodal status, VEGF-C expression, and prognosis in breast cancer. Breast Cancer Res Treat 2005; 91: 125–132.
Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 2001; 7: 192–198.
Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Ylä-Herttuala S, Jäättelä M et al. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res 2001; 61: 1786–1790.
He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T et al. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst 2002; 94: 819–825.
Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J 2001; 20: 672–682.
Roy H, Bhardwaj S, Yla-Herttuala S . Biology of vascular endothelial growth factors. FEBS Lett 2006; 580: 2879–2887.
Chen Z, Varney ML, Backora MW, Cowan K, Solheim JC, Talmadge JE et al. Down-regulation of vascular endothelial cell growth factor-C expression using small interfering RNA vectors in mammary tumors inhibits tumor lymphangiogenesis and spontaneous metastasis and enhances survival. Cancer Res 2005; 65: 9004–9011.
Morimoto J, Imai S, Haga S, Iwai Y, Iwai M, Hiroishi S et al. New murine mammary tumor cell lines. In Vitro Cell Dev Biol 1991; 27A: 349–351.
Shibata MA, Morimoto J, Otsuki Y . Suppression of murine mammary carcinoma growth and metastasis by HSVtk/GCV gene therapy using in vivo electroporation. Cancer Gene Ther 2002; 9: 16–27.
Shibata MA, Ito Y, Morimoto J, Otsuki Y . Lovastatin inhibits tumor growth and lung metastasis in mouse mammary carcinoma model: a p53-independent mitochondrial-mediated apoptotic mechanism. Carcinogenesis 2004; 25: 1887–1898.
Shibata MA, Ito Y, Morimoto J, Kusakabe K, Yoshinaka R, Otsuki Y . In vivo electrogene transfer of interleukin-12 inhibits tumor growth and lymph node and lung metastases in mouse mammary carcinomas. J Gene Med 2006; 8: 335–352.
Czauderna F, Santel A, Hinz M, Fechtner M, Durieux B, Fisch G et al. Inducible shRNA expression for application in a prostate cancer mouse model. Nucleic Acids Res 2003; 31: e127.
Zhang L, Yang N, Mohamed-Hadley A, Rubin SC, Coukos G . Vector-based RNAi, a novel tool for isoform-specific knock-down of VEGF and anti-angiogenesis gene therapy of cancer. Biochem Biophys Res Commun 2003; 303: 1169–1178.
Shibata MA, Morimoto J, Doi H, Morishima S, Naka M, Otsuki Y . Electrogene therapy using endostatin, with or without suicide gene therapy, suppresses murine mammary tumor growth and metastasis. Cancer Gene Ther 2007; 14: 268–278.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 2001; 25: 402–408.
Shibata MA, Miwa Y, Miyashita M, Morimoto J, Abe H, Otsuki Y . Electrogene transfer of an Epstein-Barr virus-based plasmid replicon vector containing the diphtheria toxin A gene suppresses mammary carcinoma growth in SCID mice. Cancer Sci 2005; 96: 434–440.
Shibata MA, Liu M-L, Knudson MC, Shibata E, Yoshidome K, Bandey T et al. Haploid loss of bax leads to accelerated mammary tumor development in C3(1)/SV40-TAg transgenic mice: reduction in protective apoptotic response at the preneoplastic stage. EMBO J 1999; 18: 2692–2701.
Gorrin-Rivas MJ, Arii S, Furutani M, Mizumoto M, Mori A, Hanaki K et al. Mouse macrophage metalloelastase gene transfer into a murine melanoma suppresses primary tumor growth by halting angiogenesis. Clin Cancer Res 2000; 6: 1647–1654.
Carter CL, Allen C, Henson DE . Relation of tumor size, lymph node status, and survival in 24 740 breast cancer cases. Cancer 1989; 63: 181–187.
Harrison R, Byrne BJ, Tung L . Electroporation-mediated gene transfer in cardiac tissue. FEBS Lett 1998; 435: 1–5.
Goto T, Nishi T, Tamura T, Dev SB, Takeshima H, Kochi M et al. Highly efficient electro-gene therapy of solid tumor by using an expression plasmid for the herpes simplex virus thymidine kinase gene. Proc Natl Acad Sci USA 2000; 97: 354–359.
Lohr F, Lo DY, Zaharoff DA, Hu K, Zhang X, Li Y et al. Effective tumor therapy with plasmid-encoded cytokines combined with in vivo electroporation. Cancer Res 2001; 61: 3281–3284.
Yamashita Y, Shimada M, Hasegawa H, Minagawa R, Rikimaru T, Hamatsu T et al. Electroporation-mediated interleukin-12 gene therapy for hepatocellular carcinoma in the mice model. Cancer Res 2001; 61: 1005–1012.
Shibata MA, Horiguchi T, Morimoto J, Otsuki Y . Massive apoptotic cell death in chemically induced rat urinary bladder carcinomas following in situ HSVtk electrogene transfer. J Gene Med 2003; 5: 219–231.
Sleeman JP . The lymph node as a bridgehead in the metastatic dissemination of tumors. Recent Results Cancer Res 2000; 157: 55–81.
Valtola R, Salven P, Heikkila P, Taipale J, Joensuu H, Rehn M et al. VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am J Pathol 1999; 154: 1381–1390.
Achen MG, Mann GB, Stacker SA . Targeting lymphangiogenesis to prevent tumour metastasis. Br J Cancer 2006; 94: 1355–1360.
Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA 1995; 92: 3566–3570.
Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 1996; 15: 290–298.
Shimizu K, Kubo H, Yamaguchi K, Kawashima K, Ueda Y, Matsuo K et al. Suppression of VEGFR-3 signaling inhibits lymph node metastasis in gastric cancer. Cancer Sci 2004; 95: 328–333.
Lin J, Lalani AS, Harding TC, Gonzalez M, Wu WW, Luan B et al. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res 2005; 65: 6901–6909.
Dvorak HF . Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 2002; 20: 4368–4380.
Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M . VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med 2005; 201: 1089–1099.
Bjorndahl MA, Cao R, Burton JB, Brakenhielm E, Religa P, Galter D et al. Vascular endothelial growth factor-a promotes peritumoral lymphangiogenesis and lymphatic metastasis. Cancer Res 2005; 65: 9261–9268.
Mercurio AM, Lipscomb EA, Bachelder RE . Non-angiogenic functions of VEGF in breast cancer. J Mammary Gland Biol Neoplasia 2005; 10: 283–290.
Roberts N, Kloos B, Cassella M, Podgrabinska S, Persaud K, Wu Y et al. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res 2006; 66: 2650–2657.
Whitehurst B, Flister MJ, Bagaitkar J, Volk L, Bivens CM, Pickett B et al. Anti-VEGF-A therapy reduces lymphatic vessel density and expression of VEGFR-3 in an orthotopic breast tumor model. Int J Cancer 2007; 121: 2181–2191.
Barbera-Guillem E, Nyhus JK, Wolford CC, Friece CR, Sampsel JW . Vascular endothelial growth factor secretion by tumor-infiltrating macrophages essentially supports tumor angiogenesis, and IgG immune complexes potentiate the process. Cancer Res 2002; 62: 7042–7049.
Valkovic T, Dobrila F, Melato M, Sasso F, Rizzardi C, Jonjic N . Correlation between vascular endothelial growth factor, angiogenesis, and tumor-associated macrophages in invasive ductal breast carcinoma. Virchows Arch 2002; 440: 583–588.
McColl BK, Stacker SA, Achen MG . Molecular regulation of the VEGF family—inducers of angiogenesis and lymphangiogenesis. Apmis 2004; 112: 463–480.
Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V et al. Vascular endothelial growth factor C induces angiogenesis in vivo. Proc Natl Acad Sci USA 1998; 95: 14389–14394.
Acknowledgements
This investigation was supported by a Grant-in-Aid for Scientific Research (C)(2) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (no. 17591360 to MA Shibata) and, in part, by a High-Tech Research Center Grant to Osaka Medical College from MEXT. We also thank Ms Hidemi Hiyama and Mika Yoshida for their excellent secretarial assistance.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shibata, MA., Morimoto, J., Shibata, E. et al. Combination therapy with short interfering RNA vectors against VEGF-C and VEGF-A suppresses lymph node and lung metastasis in a mouse immunocompetent mammary cancer model. Cancer Gene Ther 15, 776–786 (2008). https://doi.org/10.1038/cgt.2008.43
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2008.43
Keywords
This article is cited by
-
Synaptonemal complex protein 3 is associated with lymphangiogenesis in non-small cell lung cancer patients with lymph node metastasis
Journal of Translational Medicine (2017)
-
Correlation between serum levels of vascular endothelial growth factor-C and sentinel lymph node status in early breast cancer
Tumor Biology (2015)
-
Mammary cancer gene therapy targeting lymphangiogenesis: VEGF-C siRNA and soluble VEGF receptor-2, a splicing variant
Medical Molecular Morphology (2012)
-
α-Mangostin extracted from the pericarp of the mangosteen (Garcinia mangostanaLinn) reduces tumor growth and lymph node metastasis in an immunocompetent xenograft model of metastatic mammary cancer carrying a p53 mutation
BMC Medicine (2011)
-
Inhibition of cervical cancer cell growth in vitro and in vivo with dual shRNAs
Cancer Gene Therapy (2011)