Abstract
Apoptosis is induced by various stresses generated from the extracellular and intracellular environments. The fidelity of the cell cycle is monitored by surveillance mechanisms that arrest its further progression if any crucial process has not been completed or damages are sustained, and then the cells with problems undergo apoptosis. Although the molecular mechanisms involved in the regulation of the cell cycle and that of apoptosis have been elucidated, the links between them are not clear, especially that between cell cycle and death receptor-mediated apoptosis. By using the HeLa.S-Fucci (fluorescent ubiquitination-based cell cycle indicator) cells, we investigated the relationship between the cell cycle progression and apoptotic execution. To monitor apoptotic execution during cell cycle progression, we observed the cells after induction of apoptosis with time-lapse fluorescent microscopy. About 70% of Fas-mediated apoptotic cells were present at G1 phase and about 20% of cells died immediately after cytokinesis, whereas more than 60% of etoposide-induced apoptotic cells were at S/G2 phases in random culture of the cells. These results were confirmed by using synchronized culture of the cells. Furthermore, mitotic cells showed the resistance to Fas-mediated apoptosis. In conclusion, these findings suggest that apoptotic execution is dependent on cell cycle phase and Fas-mediated apoptosis preferentially occurs at G1 phase.
Similar content being viewed by others
Main
Apoptosis is a mechanism of cell death that is fundamental in many biological phenomena, including morphogenesis and maintenance of tissue homeostasis. Apoptosis is characterized by chromatin condensation, nuclear fragmentation, and formation of membrane-enclosed vesicles called apoptotic bodies, which are phagocytosed by other cells. Various stresses generated from the extracellular and intracellular environments induce apoptosis. Stimuli that trigger apoptosis in mammalian cells can be summarized into two major categories of apoptotic pathways: the extrinsic and intrinsic pathways.1, 2, 3 The extrinsic pathway occurs in response to external signals. This is also referred to as the death receptor pathway, as it is mediated by ligation of cell surface death receptors to their cognate ligands. Ligation of death receptors induces subsequent downstream signaling through the initiator caspase-8 and/or caspase-10. Intrinsic apoptotic signaling occurs in response to stimuli such as DNA damage, growth factor withdrawal, and exposure to certain chemotherapeutic agents, all of which result in the release of cytochrome c and other pro-death factors from the intermembrane space of the mitochondria and subsequent downstream signaling through the initiator caspase-9.
Fas/CD95 is a member of the tumor necrosis factor receptor superfamily, and induces apoptosis through the extrinsic pathway. The activation of Fas/CD95 by its specific ligand, FasL/CD95L, induces apoptosis in susceptible target cells.4 After activation of Fas/CD95, the adapter protein, Fas-associated protein with death domain (FADD), binds to the death domain of Fas/CD95 and attracts procaspase-8 via its death effector domain to the receptor complex, forming the death-inducing signaling complex (DISC).5, 6, 7 Upon DISC formation, procaspase-8 is autolytically cleaved and activated and, in turn, cleaves downstream caspases such as effector caspase-3 and -7, leading to cleavage of cellular proteins and DNA and to subsequence apoptotic cell death.8 On the other hand, etoposide (VP-16) is one of the most widely used anticancer drugs, belonging to the family of DNA topoisomerase II inhibitors. Etoposide causes DNA double-strand breaks through the formation of a cleavage complex containing DNA–drug–enzyme, and induces apoptosis through the intrinsic pathway.9
Cell cycle checkpoints restrain further cell cycle progression if a process has not been successfully completed or DNA damage has been sustained.10 Checkpoints operate to prevent further DNA replication within S phase when the replication complexes are stalled, to prevent entry into mitosis when DNA replication is not completed, and to prevent chromosome segregation when mitotic spindle assembly has not been completed. DNA damage-induced checkpoints also inhibit entry into S phase, progression through S phase, and entry into mitosis.
Recently, HeLa.S-Fucci (fluorescent ubiquitination-based cell cycle indicator) cells were established by Miyawaki group,11 which express monomeric Kusabira-Orange 2 (mKO2) and monomeric Azami-Green 1 (mAG1) fused to the ubiquitination domains of Cdt1 and geminin, respectively, to monitor the cell cycle progression in situ. As Cdt1 and geminin are the direct substrates of SCFSkp2 and APC/CCdh1 complexes, respectively, the level of Cdt1 is highest at G1 phase whereas geminin is prominent during S, G2, and M phases. Therefore, the cell nuclei of HeLa.S-Fucci cells during the cell cycle are labeled with orange of mKO2 fused to the ubiquitination domain of Cdt1 in G1 phase and green of mAG1 fused to the ubiquitination domain of geminin in S, G2, and M phases. The availability of the Fucci system for the analysis of tumor biology and cell biology in relation to cell cycle regulation has been reported.12, 13, 14, 15, 16
It has been unclear whether the apoptotic events are dependent on the cell cycle phase, because the available methods are not sufficient to monitor the apoptotic execution in situ. Common protocols such as immunoblotting and flow cytometry have been applied to examine the cell cycle progression and apoptotic events, which indirectly indicated the cell cycle phase of apoptotic cells by using marker proteins such as cyclins and Rb, and DNA contents.17, 18, 19, 20, 21, 22, 23, 24 However, cyclin E, Rb, and cyclin-dependent kinase (CDK) inhibitors are substrates for caspases,25, 26, 27, 28 and DNA contents gradually decrease during apoptotic execution. Furthermore, if the cells were examined after synchronization using some drugs that are toxic to cells, it is difficult to distinguish whether apoptosis is induced by apoptotic stimuli. Here we used HeLa.S-Fucci cells to directly monitor the cell cycle progression and determine the cell cycle phase of apoptotic cells induced by the extrinsic and intrinsic pathways under the time-lapse fluorescent microscopy. Our results clearly indicated that apoptotic execution is dependent on cell cycle phase and Fas-mediated apoptosis preferentially occurs at G1 phase.
Results
The ratio of cell cycle phase of normal cells after apoptosis induction
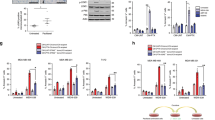
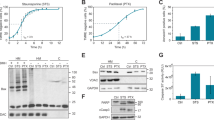
The relationship between apoptosis and the cell cycle remains unclear. Therefore, we set out to analyze the cell cycle specificity of Fas-mediated and etoposide-induced apoptotic execution using HeLa.S-Fucci cells. First, we checked the sensitivities of HeLa.S-Fucci cells to the treatment of an agonistic anti-Fas antibody and etoposide. The viability of the cells treated with the anti-Fas antibody decreased in a time-dependent manner accompanying the activation of caspases and the cleavage of caspase substrate (Figures 1a and b). The response to etoposide had a time lag of about 12 h for the apoptosis execution and caspase activation (Figures 1c and d). To study the effects of the agonistic anti-Fas antibody and etoposide on the cell cycle progression, we observed HeLa.S-Fucci cells with immunofluorescent microscope at 24 h after treatment (Figure 2a). Total number of living cells, which attached on the culture dish and showed spread shape, decreased as compared with that of non-treated cells. Although the ratio of the cells having red nuclei to the cells having green nuclei in non-treated and the anti-Fas antibody-treated cells seemed to be unchanged, the relative number of normal cells having green nuclei significantly increased after treatment with etoposide, suggesting that etoposide-induced apoptosis occurred in a cell cycle-dependent manner. To confirm this, cells showing normal morphology were counted and classified into each cell cycle phase according to the criteria as shown in Figure 2b. Cells treated with etoposide were significantly accumulated at S/G2/M phases with nuclei labeled with green color, consistent with the pharmacological action of etoposide that inhibits topoisomerase II, thereby arrests the cells at S and G2 phases (Figure 2c). Additionally, we noticed a slight decrease of cells at G1 phase accompanying an increase of cells at S/G2/M phases in the anti-Fas antibody-treated cells, suggesting that the Fas-mediated apoptosis preferentially occurs at G1 phase.
Time-course of apoptosis execution and caspase activation in HeLa.S-Fucci cells. Cells were cultured in the presence of 0.5 μg/ml of the agonistic anti-Fas antibody (a and b) or 50 μM etoposide (VP-16) (c and d) for 24 h. (a and c) Cells were observed with the time-lapse fluorescence microscope at 15-min intervals. Cell viability was assessed morphologically at each time point on the monitor of computer by counting flat and well-attached cells as viable cells, and shrunk or fragmented cells as dead cells. At least 200 cells were counted for each measurement in all experiments. The data (mean±S.D.) were obtained from at least three independent experiments. (b and d) Cell lysates were subjected to SDS−PAGE and immunoblotted with the antibodies as indicated
Effects of the treatment of the agonistic anti-Fas antibody or etoposide on cell cycle progression of HeLa.S-Fucci cells. (a) Cells were cultured in the absence (upper panels) or the presence of 0.5 μg/ml of the agonistic anti-Fas antibody (middle panels) or 50 μM etoposide (VP-16) (lower panels) for 24 h, and observed with fluorescence microscope. The morphology of the cells and the color of cell nuclei were shown by phase contrast, mKO2, mAG1, and merged images, respectively. Bars, 50 μm. (b) The criteria for the classification of normal HeLa.S-Fucci cells into each cell cycle phase. The flattened cells with mKO2 were at G1 phase, the flattened cells with both mKO2 and mAG1 were at the border of G1 and S phases, the flattened cells with mAG1 were at S and G2 phases, the smooth round cells with mAG1 were at M phase, and the smooth round or flattened cells without mKO2 nor mAG1 were at the transition from M to G1 phases. (c) The ratio of cell cycle phase of normal cells after apoptosis induction. HeLa.S-Fucci cells were cultured as described in panel a. Cells showing normal morphology were counted and classified into each cell cycle phase according to the criteria as shown in panel b. At least 100 cells were counted for each measurement in all experiments. The data (mean±S.D.) were obtained from at least three independent experiments
Time-lapse observation of Fas-mediated or etoposide-induced apoptosis
To monitor the apoptotic execution during cell cycle progression, we observed HeLa.S-Fucci cells for 24 h after treatment without or with the agonistic anti-Fas antibody or etoposide with time-lapse fluorescent microscopy (see Supplementary Movies S1–S3), and the typical features of the cells at 6 h after treatment with the anti-Fas antibody (Figure 3a) and at 24 h after treatment with etoposide (Figure 3b) were shown. Apoptotic cells with red color were marked with red arrows, apoptotic cells with green color were with green arrows, apoptotic cells with both red and green colors were with yellow arrows, and apoptotic cells without any color were with white arrows. Although most of the cells treated with etoposide had green color, suggesting that apoptotic cells were at S, G2, and M phases (Figure 3b), the majority of Fas-mediated apoptotic cells had red color indicating that the cells were dead at G1 phase (Figure 3a). To confirm this, we counted apoptotic cells and classified into each cell cycle phase according to the criteria as shown in Figure 4a. About 70% of apoptotic cells were present at G1 phase at 6 h after treatment with the anti-Fas antibody, and gradually decreased the apoptotic cells at G1 phase accompanying the increase of apoptotic cells at M/G1 phase (Figure 4b), suggesting that Fas-mediated apoptosis preferentially occurred at G1 phase. On the other hand, more than 60% of etoposide-induced apoptotic cells had green color at 24 h after treatment with etoposide (Figure 4c), indicating that the etoposide-induced apoptotic execution occurred at S/G2/M phases.
Effects of the treatment of the agonistic anti-Fas antibody or etoposide on the apoptotic execution of HeLa.S-Fucci cells. Cells were cultured in the presence of 0.5 μg/ml of the agonistic anti-Fas antibody (a) or 50 μM etoposide (VP-16) (b) for 24 h, and were observed with fluorescence microscope. The morphology of the cells and the color of cell nuclei at 6 h for Fas-treated cells (a) and at 24 h for etoposide-treated cells (b) were shown by phase contrast, mKO2, mAG1, and merged images, respectively. Apoptotic cells with mKO2 were marked with red arrows, apoptotic cells with both mKO2 and mAG1 were with yellow arrows, apoptotic cells with mAG1 were with green arrows, and apoptotic cells without any fluorescent proteins were with white arrows
Cell cycle dependency of the apoptosis execution in HeLa.S-Fucci cells treated with the agonistic anti-Fas antibody or etoposide. (a) The criteria for the classification of apoptotic HeLa.S-Fucci cells into each cell cycle phase. The shrunk cells with mKO2 were at G1 phase, the shrunk cells with both mKO2 and mAG1 were at the border of G1 and S phases, the shrunk cells with mAG1 were at S, G2, and M phases, and the shrunk cells with neither mKO2 nor mAG1 were at the transition from M to G1 phases. HeLa.S-Fucci cells were cultured in the presence of 0.5 μg/ml of the agonistic anti-Fas antibody (b) or 50 μM etoposide (VP-16) (c) for 24 h, and were observed with the time-lapse fluorescence microscope at 15-min intervals. Apoptotic cells were morphologically judged on the photographs and the movies, and were counted and classified into each cell cycle phase according to the criteria as shown in panel a. At least 100 cells were counted for each measurement in all experiments. The data (mean±S.D.) were obtained from at least three independent experiments
Preferential Fas-mediated apoptosis at G1 phase in synchronized cells
To further examine the cell cycle-dependent apoptotic execution, synchronized cells were monitored under the time-lapse fluorescent microscope (Figure 5 and Supplementary Movies S4–S6) and the numbers of normal, apoptotic, and mitotic cells were counted on the photographs from each movie. Synchronized HeLa.S-Fucci cells at the border of G1 and S phases transited through M phase from 7 to 11 h after the release to enter S phase regardless of the presence or absence of the agonistic anti-Fas antibody (Figure 5a), and the total number of cells increased (Figure 5b), suggesting that the treatment of the anti-Fas antibody did not interfere with the cell cycle progression from S to M phases. However, no mitotic cells were observed in etoposide-treated synchronized cells (Figure 5a), total number of the cells gradually decreased (Figure 5b), and a large fraction of the cells had nuclei labeling with green color (Supplementary Movie S6), suggesting that etoposide-treated cells arrested at S and/or G2 phases. The viability of synchronized cells treated with the anti-Fas antibody decreased in a time-dependent manner, but the prominent decrease was observed between 12 and 15 h after the release to enter the cell cycle (Figure 5c), indicating that the sensitivities of the cells to the treatment with the anti-Fas antibody were transiently enhanced after M phase. To confirm these results, the number of apoptotic cells per unit area was counted at each time point (Figure 5d), and the increase of apoptotic cells at each period was shown by the histogram (Figure 5e). Apoptotic cells appeared at the highest level between 12 and 15 h, suggesting that the Fas-mediated apoptosis was preferentially executed at G1 phase. In contrast, the onset of apoptotic execution of etoposide-treated cells was around 15 h after treatment, and thereafter the decrease of viability and the increase of apoptotic cells were observed in a time-dependent manner (Figures 5f–h). These results suggested that apoptotic execution is dependent on cell cycle phase and Fas-mediated apoptosis preferentially occurs at G1 phase.
Preferential apoptosis execution of HeLa.S-Fucci cells at G1 phase after treatment with the agonistic anti-Fas antibody. Cells were arrested at the border of G1 and S phase with a double-thymidine block, followed by release to enter S phase in the absence or presence of 0.5 μg/ml of the agonistic anti-Fas antibody or 50 μM etoposide (VP-16), and were observed with the time-lapse fluorescence microscope at 15-min intervals (see Supplementary Movies S4–S6). (a) Mitotic cells showing smooth round morphology with mAG1 were counted on the movies and were plotted over time. The plots showed the average number of mitotic cells from at least six fields. (b) Total cell number including normal, apoptotic, and mitotic cells were counted on the movies and the images constructing the movies. The plots showed the average number of cells from at least three fields. (c) Cell viability after treatment with the agonistic anti-Fas antibody was assessed morphologically on the movies and the images constructing the movies by counting flat and well-attached cells as viable cells, and shrunk or fragmented cells as dead cells. At least 120 cells were counted for each measurement in one movie. The data (mean±S.D.) were obtained from at least six movies. (d) The average number of apoptotic cells per one area of 0.58 mm2 at each time point was obtained from the data in panel c. (e) A histogram showed the average number of apoptotic cells per one field of 0.58 mm2. The data were obtained from panel d. (f) Cell viability after treatment with etoposide (VP-16) was assessed morphologically as described in panel c. (g) The average number of apoptotic cells per one field of 0.58 mm2 at each time point was obtained from the data in panel f. (h) A histogram showed the average number of apoptotic cells per one field of 0.58 mm2. The data were obtained from panel g
Resistance of mitotic cells to Fas-mediated apoptosis
As shown in Figures 5a and e, Fas-mediated apoptosis was transiently enhanced immediately after M phase. Furthermore, we noticed that there were no occurrence of apoptosis during M phase under time-lapse fluorescent microscope observation of both asynchronous and synchronous cells after induction of Fas-mediated apoptosis (Supplementary Movies S2 and S5). These results suggest that mitotic cells are resistant to Fas-mediated apoptosis. To confirm this, we counted mitotic cells on the movies and the images constructing the movies, and classified into normal and dead cells (Table 1). Cells treated with the agonistic anti-Fas antibody normally transited through M phase, which have more than 95% viability throughout the observation, even though the total cell viability was gradually decreased. These results strongly suggested that Fas-mediated apoptosis is not executed during M phase.
FADD, a component of DISC, was suggested to be phosphorylated at Ser-194 in arrested cells at G2/M phases.29 Therefore, we analyzed whether FADD is phosphorylated at Ser-194 during M phase (Figure 6). After synchronization of HeLa.S-Fucci cells at the border of G1 and S phases with a double-thymidine block following its release into the cell cycle, cells were collected at each time point and analyzed by immunoblotting. The doublet bands of FADD were detected at 8 and 10 h after release from the thymidine block. Furthermore, the band corresponding to phosphorylated FADD was detected with the antibody specifically recognized phosphorylated FADD at Ser-194 at the same periods. As synchronized HeLa.S-Fucci cells at the border of G1 and S phases transited through M phase from 7 to 11 h after the release to enter S phase as described in Figure 5, these results suggested that FADD is phosphorylated at Ser-194 during M phase.
FADD phosphorylation during mitotic HeLa.S-Fucci cells. Cells were arrested at the border of G1 and S phases with a double-thymidine block, followed by release to enter S phase. Cell lysates from cells at each time point were subjected to SDS–PAGE, followed by immunoblotting with antibodies as indicated. Cyclin B and cyclin E were used for cell cycle markers of G2/M and G1/S phases, respectively
Discussion
The relationship between the apoptotic execution and the cell cycle progression had been difficult to be analyzed, because it was hard to determine the cell cycle phase of each apoptotic cell. Here we determined the cell cycle phase of each apoptotic cell by using HeLa.S-Fucci cells under time-lapse fluorescent microscope observation, and showed that Fas-mediated apoptosis occurred preferentially at G1 phase whereas etoposide-induced apoptosis did at S and G2 phases.
It was suggested that the cells treated with etoposide arrested at S and/or G2 phases, as most normal and apoptotic cells had nuclei labeled with green color and no mitotic cells were observed (Figures 2, 3, 4, and Supplementary Movies S3 and S6). As etoposide forms a complex with topoisomerase II and DNA to enhance double-strand and single-strand breaks and reversible inhibition of DNA religation, the cell cycle was arrested in S and/or G2 phases by the mechanisms of the cell cycle checkpoints, leading to apoptotic cell death through the intrinsic pathway before the entry into M phase.
Fas-mediated apoptosis was executed through the extrinsic pathway of apoptosis. Although several groups tried to determine the cell cycle dependency of Fas-mediated apoptosis, the results were inconsistent: more sensitive at G1 phase,17, 21 no cell cycle dependency,18, 19 susceptible at S phase,20, 30 and dependency at G1 and S phases.22, 23 Here we clearly indicated that Fas-mediated apoptosis occurred preferentially at G1 phase. Furthermore, we noticed that no apoptosis occurred at M phase and the ratio of apoptotic cells which died immediately after cytokinesis increased in a time-dependent manner (Figure 4b and Supplementary Movies S2 and S5). These results suggest that mitotic cells show a resistance to Fas-mediated apoptosis.
How are mitotic cells resistant to Fas-mediated apoptosis? During mitosis, cells undergo dramatic changes including chromosomal condensation, nuclear envelope breakdown, and mitotic spindle formation. Phosphorylation events of multiple proteins by CDKs and other mitotic kinases are essential for the regulation of these processes.31, 32 The components of DISC were suggested to be phosphorylated during mitosis. FADD is the key adapter protein transmitting apoptotic signals mediated by the death receptors.5, 7 FADD is also implicated in cell proliferation, cell cycle progression, tumor development, inflammation, innate immunity, and autophagy.33 Ser-194 of the FADD protein has been shown to undergo phosphorylation in cells arrested in G2/M phases, and three kinases able to phosphorylate FADD at the Ser-194 residue have been identified: the 37-kDa casein kinase Iα, the 130-kDa Fas/FADD-interacting serine/threonine kinase (FIST-HIPK3), and Polo-like kinase 1.29, 34, 35, 36 Furthermore, the phosphorylation at Ser-203 of FADD by mitotic kinase Aurora-A led to cooperative phosphorylation at Ser-194 of FADD.36 In contrast to pro-apoptotic cytoplasmic FADD, the phosphorylation at Ser-194 is suggested to be essential for the nuclear localization of FADD,37 and nuclear FADD is implicated in survival mechanisms.38 As we detected the phosphorylation at Ser-194 of FADD during M phase (Figure 6), the molecular mechanisms of the resistance to Fas-mediated apoptosis in mitotic cells may be partially explained by the phosphorylation at Ser-194 of FADD that sequestered FADD molecule in nuclei thereby preventing DISC formation in the cytoplasm. Furthermore, phosphorylation at Ser-18 and Ser-21 of FADD during mitosis were identified by phosphoproteomics analysis.39 It will be necessary to evaluate the possible contribution of these phosphorylation of FADD to the regulation of Fas-mediated apoptosis. On the other hand, it was recently reported that procaspase-8 was phosphorylated in mitotic cells by Cdk1/cyclin B1 on Ser-387, which prevented Fas-mediated apoptosis.40 However, as the phosphorylated procaspase-8 at Ser-387 was predominantly localized at centrosomes from prometaphase to anaphase, further investigation will be needed to clear the relationship between the phosphorylation of procaspase-8 at Ser-387 and the resistance of mitotic cells to Fas-mediated apoptosis. Additionally, several phosphorylation of the components of DISC during mitosis was reported by phosphoproteomics analysis, such as Ser-209, Ser-212, Thr-214, and Thr-219 of Fas/CD95,41 Ser-276 and Ser-289 of procaspase-8.39 The physiological importance of the phosphorylation of these proteins in the DISC formation needs to be clarified.
Materials and Methods
Cell culture, synchronization, and apoptosis induction
HeLa.S-Fucci cells11 were cultured in DMEM supplemented with 10% fetal bovine serum (FBS). For synchronization at the border of G1 and S phases, cells were seeded at a density of 5 × 104 cells per 35-mm dish and cultured for 24 h. After exposure to 2.5 mM thymidine for 18 h, cells were washed with phosphate-buffered saline (PBS) three times and incubated in fresh medium for 10 h, and then exposed to 2.5 mM thymidine again for 14 h. To release the cells from the arrest, medium containing thymidine was removed, and the cells were washed with PBS three times and incubated in fresh medium. For induction of apoptosis, cells were treated with 0.5 μg/ml of the agonistic anti-Fas antibody (CH-11; Medical & Biological Laboratories, Nagoya, Japan) or 50 μM etoposide (VP-16).
Immunoblot analysis
Cells were lysed in lysis buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 50 μg/ml phenylmethanesulfonyl fluoride, 5 mM EDTA). Protein samples were separated by SDS–polyacrylamide gel electrophoresis and blotted onto Immobilon polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). Each protein was detected using primary antibodies as indicated, horseradish peroxidase-conjugated secondary antibodies, and ECL-plus detection reagent (GE Healthcare, Little Chalfont, UK). Anti-caspase-3 polyclonal antibody (9662), anti-caspase-8 polyclonal antibody (9764), anti-PARP polyclonal antibody (9542), and anti-phospho-FADD (Ser-194) polyclonal antibody (2781) were obtained from Cell Signaling (Danvers, MA, USA); anti-caspase-9 monoclonal antibody (M054-3), anti-FADD monoclonal antibody (M033-3), and cyclin B1 monoclonal antibody (K0128-3) were from Medical & Biological Laboratories; anti-caspase-7/MCH-3 monoclonal antibody (610812) was from BD Biosciences (Rockville, MD, USA); anti-α-tubulin monoclonal antibody (T6074) was from Sigma (St. Louis, MO, USA); anti-cyclin E monoclonal antibody (sc-247) was from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Time-lapse fluorescence microscopy
For time-lapse fluorescence microscopy, HeLa.S-Fucci cells were plated on a 35-mm dish. The medium was replaced with MEM supplemented with 10% FBS without phenol red, and dishes were placed in a humidified chamber at 37°C that was mounted on a fluorescence microscope (model BZ-8000; Keyence, Osaka, Japan) with a constant supply of mixed air containing 5% CO2. For analyses with BZ-8000, cells were observed with a 20 X objective lens and image data were obtained automatically every 15 min from seven different fields by using BZ-H1TL software (Keyence). In the original program by the manufacturer, cells are exposed to the intense mercury lamp light intermittently and the cells were driven to apoptosis within the initial 6 h by the physical damage of the light. Therefore, we used the neutral density filters to reduce the light levels to 1.6% that allowed the cells to proliferate during 48 h observation.42
Abbreviations
- Fucci:
-
fluorescent ubiquitination-based cell cycle indicator
- FADD:
-
Fas-associated protein with death domain
- DISC:
-
death-inducing signaling complex
- mKO2:
-
monomeric Kusabira-Orange 2
- mAG1:
-
monomeric Azami-Green 1
References
Thornberry NA, Lazebnik Y . Caspases: enemies within. Science 1998; 281: 1312–1316.
Earnshaw WC, Martins LM, Kaufmann SH . Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 1999; 68: 383–424.
Riedl SJ, Shi Y . Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol 2004; 5: 897–907.
Nagata S . Apoptosis by death factor. Cell 1997; 88: 355–365.
Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J 1995; 14: 5579–5588.
Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D . A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem 1995; 270: 7795–7798.
Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM . FADD a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 1995; 81: 505–512.
Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J 1998; 17: 1675–1687.
Baldwin EL, Osheroff N . Etoposide topoisomerase II and cancer. Curr Med Chem Anticancer Agents 2005; 5: 363–372.
Clarke PR, Allan LA . Cell-cycle control in the face of damage-a matter of life or death. Trends Cell Biol 2009; 19: 89–98.
Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 2008; 132: 487–498.
Sakaue-Sawano A, Kobayashi T, Ohtawa K, Miyawaki A . Drug-induced cell cycle modulation leading to cell-cycle arrest, nuclear mis-segregation, or endoreplication. BMC Cell Biol 2011; 12: 2.
Hashimoto T, Kikkawa U, Kamada S . Contribution of caspase(s) to the cell cycle regulation at mitotic phase. PLoS One 2011; 6: e18449.
Nakayama M, Kaida A, Deguchi S, Sakaguchi K, Miura M . Radiosensitivity of early and late M-phase HeLa cells isolated by a combination of fluorescent ubiquitination-based cell cycle indicator (Fucci) and mitotic shake-off. Radiat Res 2011; 176: 407–411.
Kaida A, Miura M . Visualizing the effect of hypoxia on fluorescence kinetics in living HeLa cells using the fluorescent ubiquitination-based cell cycle indicator (Fucci). Exp Cell Res 2012; 318: 288–297.
Dan S, Okamura M, Mukai Y, Yoshimi H, Inoue Y, Hanyu A et al. ZSTK474, a specific phosphatidylinositol 3-kinase inhibitor, induces G1 arrest of the cell cycle in vivo. Eur J Cancer 2012; 48: 936–943.
Beletskaya IV, Nikonova LV, Beletsky IV . Cell cycle specificity of Fas-mediated apoptosis in WIL-2 cells. FEBS Lett 1997; 412: 91–93.
Dao T, Huleatt JW, Hingorani R, Crispe IN . Specific resistance of T cells to CD95-induced apoptosis during S phase of the cell cycle. J Immunol 1997; 159: 4261–4267.
Hueber A, Durka S, Weller M . CD95-mediated apoptosis: no variation in cellular sensitivity during cell cycle progression. FEBS Lett 1998; 432: 155–1557.
N′cho M, Brahmi Z . Evidence that Fas-induced apoptosis leads to S phase arrest. Hum Immunol 2001; 62: 310–319.
He X, Hu J, Li X, Xiao H, Tao D, Long H et al. The approaches in detecting cell cycle specificity of Fas-mediated apoptosis in leukemia cell lines and activated PBLs in vitro. Chinese German J Clin Oncol 2006; 5: 379–382.
Feng Y, Wu J, Feng X, Tao D, Hu J, Qin J et al. Timing of apoptosis onset depends on cell cycle progression in peripheral blood lymphocytes and lymphocytic leukemia cells. Oncol Rep 2007; 17: 1437–1444.
Li Y, Dida F, Iwao A, Deguchi T, Azuma E, Komada Y . Cell cycle dependency of caspase activation in Fas-induced apoptosis in leukemia cells. Cancer Sci 2007; 98: 1174–1183.
Pundt N, Peters MA, Wunrau C, Strietholt S, Fehrmann C, Neugebauer K et al. Susceptibility of rheumatoid arthritis synovial fibroblasts to FasL- and TRAIL-induced apoptosis is cell cycle-dependent. Arthritis Res Ther 2009; 11: R16.
Mazumder S, Gong B, Chen Q, Drazba JA, Buchsbaum JC, Almasan A . Proteolytic cleavage of cyclin E leads to inactivation of associated kinase activity and amplification of apoptosis in hematopoietic cells. Mol Cell Biol 2002; 22: 2398–2409.
Jänicke RU, Walker PA, Lin XY, Porter AG . Specific cleavage of the retinoblastoma protein by an ICE-like protease in apoptosis. EMBO J 1996; 15: 6969–6978.
Gervais JL, Seth P, Zhang H . Cleavage of CDK inhibitor p21(Cip1/Waf1) by caspases is an early event during DNA damage-induced apoptosis. J Biol Chem 1998; 273: 19207–19212.
Levkau B, Koyama H, Raines EW, Clurman BE, Herren B, Orth K et al. Cleavage of p21Cip1/Waf1 and p27Kip1 mediates apoptosis in endothelial cells through activation of Cdk2: role of a caspase cascade. Mol Cell 1998; 1: 553–563.
Scaffidi C, Volkland J, Blomberg I, Hoffmann I, Krammer PH, Peter ME . Phosphorylation of FADD/ MORT1 at serine 194 and association with a 70-kDa cell cycle-regulated protein kinase. J Immunol 2000; 164: 1236–1242.
Algeciras-Schimnich A, Griffith TH, Lynch DH, Paya CV . Cell cycle-dependent regulation of FLIP levels and susceptibility to Fas-mediated apoptosis. J Immunol 1999; 162: 5205–5211.
Pines J . Mitosis: a matter of getting rid of the right protein at the right time. Trends Cell Biol 2006; 16: 55–63.
Sullivan M, Morgan DO . Finishing mitosis, one step at a time. Nat Rev Mol Cell Biol 2007; 11: 894–903.
Tourneur L, Chiocchia G . FADD: a regulator of life and death. Trends Immunol 2010; 7: 260–269.
Alappat EC, Feig C, Boyerinas B, Volkland J, Samuels M, Murmann AE et al. Phosphorylation of FADD at serine 194 by CKIα regulates its nonapoptotic activities. Mol Cell 2005; 19: 321–332.
Rochat-Steiner V, Becker K, Micheau O, Schneider P, Burns K, Tschopp J . FIST/HIPK3: a Fas/FADD-interacting serine/threonine kinase that induces FADD phosphorylation and inhibits fas-mediated Jun NH(2)-terminal kinase activation. J Exp Med 2000; 192: 1165–1174.
Jang MS, Lee SJ, Kang NS, Kim E . Cooperative phosphorylation of FADD by Aur-A and Plk1 in response to Taxol triggers both apoptotic and necrotic cell death. Cancer Res 2011; 71: 7207–7215.
Screaton RA, Kiessling S, Sansom OJ, Millar CB, Maddison K, Bird A et al. Fas-associated death domain protein interacts with methyl-CpG binding domain protein 4: a potential link between genome surveillance and apoptosis. Proc Natl Acad Sci USA 2003; 100: 5211–5216.
Gómez-Angelats M, Cidlowski JA . Molecular evidence for the nuclear localization of FADD. Cell Death Differ 2003; 10: 791–797.
Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal 2010; 3: ra3.
Matthess Y, Raab M, Sanhaji M, Lavrik IN, Strebhardt K . Cdk1/cyclin B1 controls Fas-mediated apoptosis by regulating caspase-8 activity. Mol Cell Biol 2010; 30: 5726–5740.
Dephoure N, Zhou C, Villén J, Beausoleil SA, Bakalarski CE, Elledge SJ et al. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci USA 2008; 105: 10762–10767.
Hashimoto T, Yamauchi L, Hunter T, Kikkawa U, Kamada S . Possible involvement of caspase-7 in cell cycle progression at mitosis. Genes Cells 2008; 13: 609–621.
Acknowledgements
We thank Dr. Atsushi Miyawaki for HeLa.S-Fucci cells. This work was supported in part by Grant-in-Aid for Scientific Research on Priority Areas of the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by Grant-in-Aid for Scientific Research (B) of Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by A Stephanou
Supplementary Information accompanies the paper on Cell Death and Disease website
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Hashimoto, T., Juso, K., Nakano, M. et al. Preferential Fas-mediated apoptotic execution at G1 phase: the resistance of mitotic cells to the cell death. Cell Death Dis 3, e313 (2012). https://doi.org/10.1038/cddis.2012.52
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2012.52