Abstract
Terminal differentiation of B cells depends on two interconnected survival pathways, elicited by the B-cell receptor (BCR) and the BAFF receptor (BAFF-R), respectively. Loss of either signaling pathway arrests B-cell development. Although BCR-dependent survival depends mainly on the activation of the v-AKT murine thymoma viral oncogene homolog 1 (AKT)/PI3-kinase network, BAFF/BAFF-R-mediated survival engages non-canonical NF-κB signaling as well as MAPK/extracellular-signal regulated kinase and AKT/PI3-kinase modules to allow proper B-cell development. Plasma cell survival, however, is independent of BAFF-R and regulated by APRIL that signals NF-κB activation via alternative receptors, that is, transmembrane activator and CAML interactor (TACI) or B-cell maturation (BCMA). All these complex signaling events are believed to secure survival by increased expression of anti-apoptotic B-cell lymphoma 2 (Bcl2) family proteins in developing and mature B cells. Curiously, how lack of BAFF- or APRIL-mediated signaling triggers B-cell apoptosis remains largely unexplored. Here, we show that two pro-apoptotic members of the ‘Bcl2 homology domain 3-only’ subgroup of the Bcl2 family, Bcl2 interacting mediator of cell death (Bim) and Bcl2 modifying factor (Bmf), mediate apoptosis in the context of TACI-Ig overexpression that effectively neutralizes BAFF as well as APRIL. Surprisingly, although Bcl2 overexpression triggers B-cell hyperplasia exceeding the one observed in Bim−/−Bmf−/− mice, Bcl2 transgenic B cells remain susceptible to the effects of TACI-Ig expression in vivo, leading to ameliorated pathology in Vav-Bcl2 transgenic mice. Together, our findings shed new light on the molecular machinery restricting B-cell survival during development, normal homeostasis and under pathological conditions. Our data further suggest that Bcl2 antagonists might improve the potency of BAFF/APRIL-depletion strategies in B-cell-driven pathologies.
Similar content being viewed by others
Main
Naïve B cells depend on B-cell receptor (BCR)-tuned survival signals that allow them to egress from bone marrow and complete differentiation in the spleen via different transitional (T) stages.1, 2, 3 Once in the spleen, autoreactivity of expressed BCRs is controlled again at the transitional T1 stage and survivors develop via the T2 stage into follicular (FO) or marginal zone (MZ) B cells, ready for antigen encounter.3, 4 MZ B cells together with innate-like B1 B cells from spleen and coelomic cavities are responsible for the production of natural immunoglobulins (Ig) and T cell-independent antibody responses, leading to the production of low-affinity IgM and IgG, whereas FO B cells can mature into class-switched Ig-secreting plasma or memory B cells in germinal center reactions during adaptive immune responses.5
Although B-cell homeostasis was thought to rely exclusively on tonic BCR signaling,3, 6 this view changed upon the discovery that deletion or neutralization of the B-cell survival factor, BAFF/BlyS/TALL-1/zTNF47, 8 or the receptor BAFF-R/BR3, arrested B-cell development at the transitional T1 stage.9, 10 The TNF family cytokine BAFF signals mainly via two receptors, above-mentioned BAFF-R and transmembrane activator and CAML interactor (TACI), the latter also transmitting signals from a related TNF family cytokine, APRIL, that can again selectively engage an alternative receptor, B-cell maturation (BCMA), shown to be required for plasma cell survival.11, 12, 13 Notably, neutralization of BAFF, by injection or transgenic expression of IgG1-Fc receptor-fusion proteins of the BAFF-R or TACI, causes the loss of B cells from the T2 maturation stage onwards in mice, whereas BCMA-IgG1-Fc overexpression had no effect,8, 14 defining the BAFF/BAFF-R axis as key for normal B-cell development.
Heterozygous mutations in TACI are causally linked to IgA and common variable immune deficiencies (CVIDs) in humans, characterized by antibody deficiencies, B lymphopenia and autoimmune manifestations.15 Similarly, homozygous BAFF-R mutations cause CVID in conjunction with severe B-cell deficiency.16 Targeting excess BAFF by neutralizing antibodies or recombinant receptor-fusion proteins has been tested in clinical trials for their efficacy to treat Sjögren syndrome, rheumatoid arthritis or systemic lupus erythematosus (SLE), yet results in clinical settings were not always satisfactory. Second use for some of these reagents is considered for the treatment of certain B-cell malignancies including follicular lymphoma or chronic lymphocytic leukemia and one such drug has entered phase II/III clinical trials for the treatment of pre-treated multiple myeloma.17
BAFF is thought to inhibit B-cell death mainly by activating non-canonical NF-κB signaling, ultimately leading to the transcriptional induction of pro-survival members of the B-cell lymphoma 2 (Bcl2) family and known NF-κB targets, such as Bcl2 itself,18 Bcl2-related protein X (BclX)19 or Bfl1/A1.20 However, BAFF-R activation also leads to increased v-AKT murine thymoma viral oncogene homolog 1 (AKT) and extracellular-signal regulated kinase (ERK) activity that can act on Mcl1 protein stability.21, 22 Notably, absence of Bcl223 or Mcl124 or A1 knockdown25 coincides with B-cell loss, whereas overexpression of BAFF or Bcl2 associates with B-cell hyperplasia leading to signs of SLE-like disease in mice.11, 26 Consistently, overexpression of Bcl29 or BclX27 can rescue B-cell development in the absence of BAFF signaling, albeit for reasons unclear, only partially. APRIL, which signals via the BCMA receptor for plasma cell survival, also activates NF-κB signaling and is believed to act mainly via induction of Mcl1, whereas BclX appears dispensable.28
Deprivation of BAFF or APRIL signaling promotes apoptosis, but the molecular details remain largely undefined. One study addressed the link between Bcl2 interacting mediator of cell death (Bim), BAFF and autoimmunity in vitro demonstrating that BAFF signaling actually counteracts IgM-driven B-cell apoptosis by promoting ERK-dependent proteasomal degradation of Bim in the WEHI-231 B cell system.29 Autoreactive B cells appear to depend on increased BAFF signaling for survival to counteract self-antigen-driven increases in Bim levels.30 The reduced BAFF responsiveness of Bim-deficient B cells and accumulation of normal as well as autoreactive anti-HEL-specific B cells in Bim−/− and autoantigen-exposed Bim−/−HEL-BCR transgenic mice further points to a prominent role for Bim in this process.31, 32 Together, this supports the idea that BAFF controls B-cell survival by reducing Bim activity and/or simultaneous induction of Bcl2 pro-survival homologs. Similarly, Bim-deficient mice show increased plasma cell numbers and elevated serum Ig-titers, suggesting a role in limiting their survival by antagonizing Mcl1, downstream of APRIL/BCMA.28, 33 However, direct genetic evidence defining Bim as the critical mediator of B-cell death in the absence of BAFF or APRIL is lacking and redundancy with other Bcl2 homology domain (BH) 3-only proteins can be expected.34 Hence, we explored the genetic determinants of B-cell death caused by BAFF and APRIL depletion by analyzing expression levels of Bcl2 family proteins in developing B cells under normal conditions as well as in response to chronic BAFF and APRIL deprivation and by testing the functional relevance of BH3-only proteins constitutively expressed in B cells by genetic co-deletion experiments in TACI-Ig transgenic mice.
Results
TACI-Ig expression triggers Bcl2 regulated B-cell death
We noted increased apoptosis in BAFF-sensitive T2 and FO B-cell subsets derived from TACI-Ig mice that lack bioavailable BAFF and APRIL, the latter dispensable for normal B-cell development.8 MZ B cells from TACI-Ig mice also showed increased apoptosis rates, but strong biological variation precluded establishment of significant differences. In contrast, BAFF-independent T1 B cells appeared unaffected (Supplementary Figure 1 and Figure 1a). When put in culture, however, only minor differences were noted, suggesting that all these B-cell subsets do have a comparable propensity to undergo spontaneous apoptosis ex vivo (Figure 1b). In contrast, cells from TACI-Ig mice that simultaneously overexpressed Bcl2 were completely protected from apoptosis (Figures 1a and b). Hence, we concluded that lack of BAFF primes B cells to Bcl2-regulated mitochondrial apoptosis in situ.
Increased B-cell apoptosis in the absence of BAFF signaling. (a) Splenocytes derived from mice of the indicated genotypes were stained with fluorochrome-labeled monoclonal antibodies to distinguish the different B-cell subsets, that is, T1 (IgM+CD21−CD23−), T2 (IgM+CD21highCD23+), FO (CD23+CD21+) and MZ (CD23−CD21+). Cell death was monitored by Annexin V/7-AAD staining and flow cytometric analysis. Bars represent means of four to seven independent animals per genotype±S.E.M. (b) Sorted B-cell subsets were put in culture and cell death was determined after 24 h by Annexin V/7-AAD staining and flow cytometric analysis. Bars represent means±S.E.M. from three individual experiments. Significant differences compared with wt cells are marked with *P<0.05; **P<0.005
Chronic BAFF and APRIL deprivation has only a minor impact on Bcl2 family expression
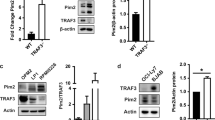
Comparison of the relative expression of Bcl2 family proteins in T1, T2, MZ and FO B cells, sorted from spleens of wt or TACI-Ig mice, that contain only few residual B-cells (Supplementary Figure 1), by reverse transcription-mixed ligation probe amplification (RT-MLPA)35 indicated solely mild changes in mRNA levels (Figure 2a). Notably, Bmf mRNA was found mildly increased in T2 and MZ cells isolated from TACI-Ig mice, whereas levels of Bid were reduced in BAFF-independent T1 cells. Contrasting our hypothesis, anti-apoptotic A1 was found increased in MZ and FO B cells from TACI-Ig mice (Figure 2a). We also quantified mRNA levels of pro-apoptotic Bcl2 proteins implicated in B-cell homeostasis by qRT-PCR, but failed to notice significant differences (Supplementary Figure 2A). Similarly, mRNA levels of Bcl2, Mcl1 or A1 were not statistically different in FO B cells isolated from wt or TACI-Ig mice (Supplementary Figure 2A).
Influence of TACI-Ig expression on Bcl2 family expression. (a) Different B-cell subsets were sorted from wt and TACI-Ig mice for RNA extraction and subsequent RT-MLPA analysis. Bars represent means of relative mRNA expression levels of n=3 mice per genotype ±S.E.M. Significant differences compared with wt cells are marked with *P<0.05. (b) B-cell subsets were isolated from the spleen of wt or TACI-Ig mice. Cells from three wt and four to seven TACI-Ig mice, depending on the subset, were pooled per lane (50 μg total protein). Two independent sorting experiments were performed and analyzed simultaneously by SDS-PAGE. Membranes were probed, stripped and re-probed sequentially, anti-tubulin was used to control for protein loading. Bmf is expressed as a long and a short variant, described in Datta et al48
As Bcl2 family proteins are influenced by post-translational modification in abundance and function,36 we decided to sort different B-cell types from wt mice and the residual cells found in TACI-Ig mice expressing the relevant surface markers for western blotting analyses. Surprisingly, in cells of TACI-Ig mice, there was a strong decrease in Bim protein in T1 B cells and lower Bim levels were also observed in T2 and FO B cells, albeit the effect was clearly not as pronounced. In addition, pro-apoptotic proteins Bcl2 antagonist of cell death (Bad) and Bmf were increased in MZ B cells from TACI-Ig mice, consistent with our RT-MLPA data. Of note, Bcl2 and Mcl1 were reduced in FO B cells derived from TACI-Ig mice that may render these cells more susceptible to apoptosis (Figure 2b). To exclude the possibility that B cells surviving in a TACI-Ig transgenic environment may have adopted their Bcl2 rheostat, we also isolated follicular B cells from wt mice and cultured them in the absence or presence of BAFF. Although levels of Bim, Mcl1 and Bcl2 changed over time, none of these changes was BAFF dependent (Supplementary Figure 2B). Together, this documents that chronic BAFF (and/or APRIL) depletion causes minimal changes in the expression profile of individual Bcl2 family proteins.
Loss of BH3-only proteins Bim or Bmf reduces BAFF-responsiveness of B cells
As our expression analysis did not unambiguously identify the relevant pro-apoptotic effector(s) of B-cell death in the absence of BAFF, we sorted different B-cell subsets from wt mice or BH3-only protein mouse mutants and analyzed their viability in culture. This analysis revealed that only FO B cells from Bim−/− or Vav-Bcl2 transgenic mice were protected from cell death, whereas those lacking Bmf, Bad, p53-upregulated modulator of apoptosis (Puma) or Noxa behaved like wt (Figure 3a; left column). Similar findings were made in T2 and MZ B cells and again Bcl2 overexpression was superior to loss of Bim in preventing B-cell apoptosis ex vivo (Supplementary Figure 3). Assessing BAFF responsiveness it became obvious that B cells overexpressing Bcl2 were completely refractory to the cytokine (Figure 3a; right column). However, Bim−/− cells still benefited from exogenous BAFF, suggesting that they are partially responsive to this cytokine. FO B cells lacking Bmf also showed an ameliorated response, suggesting that Bmf may be a possible additional target of BAFF-R signaling (Figure 3a). These findings were recapitulated to a large degree in T2 and MZ B cells (Supplementary Figure 3).
Impact of BH3-only protein deficiency on cell death and BAFF responsiveness. (a) FO B cells were sorted from the spleen of different BH3-only protein knockout or Bcl2 transgenic mice and put in culture in the presence or absence of 10 ng/ml recombinant mBAFF. Viability was assessed by AnnexinV/PI staining and flow cytometry. Bars represent means ±S.E.M. of n=4 independent experiments. Significant differences compared with wt cells are marked with *P<0.05; **P<0.005. The percentage of increased survival reflects the viability of BAFF treated minus the viability of untreated cells. (b) For titration, graded concentrations of human or mouse TACI-Ig were injected i.p. at day 0 and 4 into wt mice (left panel). The percentage of splenic B cells from treated, compared with PBS injected control mice was assessed on day 7 by flow cytometric analysis (right panel). Bars represent means of two to four mice±S.E.M. For the actual experiment, mice of the indicated genotypes were treated with 10 μg hTACI-Ig administrated i.p. on day 0 and day 4. Mice were killed on day 7 and the percentage of splenic FO B cells (CD23+CD21+) was analyzed in the spleen by flow cytometric analysis (right panel). Bars represent means±S.E.M. from three to five mice per genotype. Significant differences are marked with *P<0.05
To consolidate these observations, we tested the BH3-only protein knockout mice with reported phenotypes in the B-cell compartment37, 38, 39, 40 for their response to recombinant TACI-Ig injection. After initial titration experiments using either recombinant human or mouse TACI-Ig (Figure 3b, left panel), we injected saline, hIgG1 or 10 μg hTACI-Ig fusion protein, that is, the same protein expressed in TACI-Ig mice, following published procedure.41 In line with our in vitro observations, FO B cells from Vav-Bcl2, Bmf−/− or Bim−/− mice proved resistant to this treatment, whereas wt, Puma−/− or Bad−/− mice displayed significant B-cell loss (Figure 3b, right panel, Supplementary Figure 1H). Based on these findings, we anticipated essential roles for Bim and Bmf in B-cell death in the absence of BAFF and therefore inter-crossed TACI-Ig mice with Bim−/− or Bmf−/− mice and compared them to TACI-Ig/Vav-Bcl2 mice.
Loss of Bim and Bmf prevents B-cell death in TACI-Ig transgenic mice
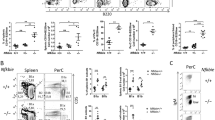
Spontaneous apoptosis of different B-cell subsets was quantified immediately after killing the mice using combined AnnexinV and B-cell surface marker staining of splenocytes. Analysis of T1 B cells revealed no relevant differences in the percentage of apoptotic cells in the absence or presence of soluble TACI-Ig (Figure 4a). In contrast, and as noted before, BAFF responsive T2 and FO B cells showed increased apoptosis in the TACI-Ig background, when compared with wt. Surprisingly, Bmf deficiency did not seem to confer protection from BAFF deprivation, presenting with comparable apoptosis rates in situ. Bim−/− cells, however, showed no increase in cell death, similar to B cells from Bim−/−Bmf−/− or Vav-Bcl2 mice. MZ B cells showed a similar trend but strong overall variation due to high background affinity for AnnexinV (Figure 4a).
Loss of Bim plus Bmf rescues B-cell loss in TACI-Ig transgenic mice. (a) Splenocytes derived from mice of the indicated genotypes were stained with relevant fluorochrome-labeled monoclonal antibodies to distinguish T1 (IgM+ CD21−), T2 (IgM+ CD21+), FO (CD23+ CD21+) and MZ (CD23− CD21+) B cells. Cell death in situ was monitored by Annexin V/7-AAD staining and flow cytometric analysis. (b) Different B-cell subsets were identified by flow cytometric analysis. T1 (IgM+CD21−CD23−), T2 (IgM+CD21+CD23−), FO (CD21+CD23+), MZ (IgD+gMlowCD93−CD1d+), PC (CD138+IgM−/low). Relative percentages and total splenic cellularities were used to calculate the absolute numbers of different B-cell subsets. Bars represent numbers 8–11±S.E.M. Significant differences are marked with *P<0.05; **P<0.005 and are compared with the genotypes±the TACI-Ig. # indicates significant differences P<0.05 between, wt, Bmf- and Bim-mutant mice expressing TACI-Ig
Comparison of the hematopoietic system of compound mutant animals revealed numerous changes. Although the overall bone marrow cellularity was not significantly different, we confirmed a significant reduction in recirculating mature B cells in the TACI-Ig mice (Supplementary Table 1). This loss was less severe in the TACI-Ig mice lacking either Bim or Bmf and completely rescued when both proteins were missing, or when Bcl2 was overexpressed (Supplementary Table 1).
Unexpectedly, T1 B-cell numbers in the spleen, considered BAFF-independent, were also significantly reduced in TACI-Ig mice and this effect was alleviated by either loss of Bim, Bmf, both or Bcl2 overexpression (Figure 4b and Supplementary Table 2). BAFF-dependent stages, that is, T2, FO and MZ, were strongly reduced in number by TACI-Ig expression, an effect less pronounced on a Bim or Bmf-deficient background. Strikingly, B-cell numbers were no longer significantly different, regardless of absence or presence of TACI-Ig, in DKO mice (Figure 4b). Notably, Bcl2 transgenic mice accumulated even more B cells than DKO mice, but in contrast to these animals, TACI-Ig co-expression reduced the number of T2, FO and MZ B cells to a significant degree (Figure 4b and Supplementary Table 2). Short-lived plasma cells in the spleen were clearly elevated above wt levels in Bim−/−, DKO and Bcl2 transgenic mice and TACI-Ig expression reduced their number in a Bim-dependent manner (Figure 4b).
Inguinal lymph nodes of TACI-Ig mice showed about 90% reduction in B-cell numbers (Supplementary Table 3). As in the spleen, single deficiency for Bim or Bmf reduced B-cell loss, whereas Bim−/−Bmf−/− animals showed no response to BAFF deprivation in vivo, a finding also observed in peripheral blood (Supplementary Table 3).
B1 ‘innate-like’ B cells do develop in the absence of BAFF signaling but express the BAFF-R as well as TACI and respond to BAFF with increased survival and enhanced proliferation ex vivo.42 Hence, we assessed B1a and B1b B-cell numbers in the peritoneal cavity of TACI-Ig mice and found them to be reduced to about 25% in number when compared with wt, contrasting previous reports.7, 8 The reduction of B1 cells was less pronounced in the absence of Bmf or Bim and again fully rescued on a double-deficient background. Similar to our findings in the spleen and lymph node, Bcl2 overexpression provided only partial protection (Supplementary Table 4).
As expected, the numbers of Mac1+ myeloid or Ter119+ nucleated erythroid cells were not affected by TACI-Ig expression (Supplementary Table 1). Surprisingly, T cells in the spleen and lymph node of TACI-Ig mice were underrepresented when compared with wt controls (Supplementary Table 2). This effect was ameliorated by loss of Bim or Bmf and completely compensated for when both genes were lost, suggesting that B-cell-derived factors or signals are needed to support T-cell homeostasis in vivo. Of note, the effects on B-cell homeostasis were highly specific for deletion of Bim and/or Bmf, as TACI-Ig mice lacking Puma or Bim plus Puma did not show any of the rescue phenotypes resembling those caused by Bmf deficiency or exceeding those caused by Bim deficiency (Supplementary Table 5). Hence, we conclude that BAFF secures B-cell survival by selectively neutralizing the pro-apoptotic function of Bim and Bmf in B1 and B2 B cells.
Bim and Bmf deficiency confer a B-cell-autonomous survival advantage
It remained formally possible that Bim- and/or Bmf-deficient non-hematopoietic cells such as intestinal epithelial cells or adipocytes17 contribute to improved B-cell survival either by producing increased BAFF levels, outcompeting the capacity of TACI-Ig, or by providing alternative B-cell extrinsic survival factors that may compensate for the loss of BAFF. However, using a cell death reporter assay to determine the presence of bioavailable TACI-Ig in serum8 revealed sufficient levels of the transgene to buffer exogenously added BAFF in all genotypes analyzed (Supplementary Figure 4a). Nonetheless, as serum level may not faithfully reflect TACI-Ig levels in the niches where B cells develop, we also reconstituted lethally irradiated co-isogenic TACI-Ig transgenic mice with bone marrow from wt, Bmf−/−, Bim−/− or DKO mice. Mice were analyzed 12 weeks after reconstitution and this revealed that indeed significantly more B cells accumulated in TACI-Ig recipients when donor bone marrow lacking Bmf, Bim, both or overexpressing Bcl2 was used (Supplementary Figure 4b). Together with our acute TACI-Ig depletion experiments (Figure 3b), this confirms that combined Bim and Bmf deficiency does provide a cell autonomous survival advantage to B cells in the absence of BAFF.
Bim and Bmf loss enables the development of antigen-competent B cells in TACI-Ig mice
To assess if the B cells accumulating in the absence of Bim and/or Bmf upon BAFF and APRIL deprivation in vivo are also functional, we immunized mice with NP-OVA adsorbed to alum to induce a T cell-dependent immune response. Alternatively, animals were immunized with the T cell-independent antigen TNP-Ficoll to interrogate the functionality of developing MZ and B1 B cells. These analyses confirmed reduced levels of NP- and TNP-specific Ig levels in TACI-Ig mice. Loss of Bmf had only limited impact on NP-specific Ig-titers, loss of Bim partially restored and combined loss of Bim plus Bmf, similar to Bcl2 overexpression, fully restored NP-specific antibody production (Figure 5). Of note, double deficiency also efficiently restored T-independent immune responses, indicating that the surviving B cells in Bim−/−Bmf−/− mice were indeed functional in the presence of TACI-Ig (Supplementary Figure 5).
Restoration of Ig production in TACI-Ig mice by cell death inhibition. Mice of the indicated genotypes were immunized with NP-Ovalbumin and sera were taken on day 10. NP-specific Ig-titers were measured by ELISA. Dilutions were predetermined to produce absorbance readings in the linear range. Total Ig (1 : 3200), IgM (1 : 200), IgG1 (1 : 25 000), IgG2a and IgG2b (1 : 3200), IgG3 (1 : 100). Box blots represent values from five to nine per mice per genotype. Box length equals interquartile range. Circles represent minimal and maximal ranges. Significant differences are marked with *P<0.05, **P<0.005 and are compared to the according genotype without the TACI-Ig
TACI-Ig expression reduces disease burden in Vav-Bcl2 mice
In Vav-Bcl2 mice, transgene overexpression leads to premature death due to development of follicular lymphoma or autoimmune glomerulonephritis.43 Although Bcl2 overexpressing B cells were highly apoptosis resistant and completely refractory to exogenous BAFF in culture (Figures 1 and 3), we noted that expression of TACI-Ig led to reduced B-cell numbers in the spleen, lymph nodes and peritoneal cavity of Vav-Bcl2 mice. This contrasted findings made in Bim−/−Bmf−/− mice, in which B-cell numbers were equally elevated in the presence or absence of TACI-Ig transgene expression (Figure 4b and Supplementary Tables 2–4). Hence, we reasoned that Bcl2 overexpression may prime B cells to death by sequestering large amounts of BH3-only proteins, such as Bim,44 that can become activated upon BAFF deprivation and may be released by sensitizers, such as Bmf or Bad, to trigger apoptosis. To mimic this situation, FO B cells from Vav-Bcl2 mice and wt controls were exposed to graded doses of the BH3-mimetic ABT-737 ex vivo, mimicking the function of Bmf or Bad.45 Although Bcl2 transgenic FO B cells did not respond to factor deprivation, these cells were highly sensitive to ABT-737 and died as fast as wt cells under these conditions. Consistent with our hypothesis, B cells lacking Bim and Bmf were highly resistant to spontaneous death and to ABT-737 (Supplementary Figure 6).
As BAFF-neutralizing reagents are used to treat SLE and considered as therapy for different B-cell malignancies,17 we tested if TACI-Ig would ameliorate pathology in Vav-Bcl2 mice. Hence, we monitored the disease-free survival of wt, TACI-Ig, Vav-Bcl2 and double-transgenic mice and took serum samples after 2, 8 or 12 months. Vav-Bcl2 mice showed the well-known pathological increase in total serum Ig,43, 46 a phenotype that was significantly reduced in the double-transgenic mice at 2 and 8 months but no longer different in mice that survived for 1 year (Figure 6a). Despite this gradual adaptation, double-transgenic mice showed extended disease-free survival (Figure 6c) and histological assessment of spleens of phenotypically healthy mice killed after 8 or 12 months showed clearly improved splenic architecture, reduced follicle and germinal center size and no lymphatic infiltrates in the liver parenchyma, in clear contrast to findings made in age-matched Vav-Bcl2 mice (Figure 7a, not shown). To find out if the survival of autoantibody-secreting plasma cells may be reduced in the presence of TACI-Ig, we also quantified dsDNA-specific autoantibodies in sera of aged single- and double-transgenic mice. However, these were found to be comparable by ELISA (Figure 6b). Immunohistochemistry revealed glomerular Ig deposits in the kidneys of single- and double-transgenic animals, albeit with reduced intensity in the latter (Figure 7b). Based on these observations, we conclude that the prolonged survival of double-transgenic mice is most likely due to a reduced severity of SLE-like symptoms combined with a reduced risk to develop follicular lymphoma.
Expression of the TACI-Ig reduces disease burden and extends the lifespan of Vav-Bcl2 mice. (a) Levels of total Ig in sera from mice of the indicated genotypes were determined at the age of 2, 8 or 12 months by ELISA. Dilutions were predetermined to produce absorbance readings in the linear range (1 : 3200). (b) Levels of anti-ds-DNA antibodies were quantified in the surviving 8- or 12-month-old mice presenting without overt signs of disease. Box blots represent values from five to nine animals per genotype. Box length equals interquartile range. Circles represent minimal and maximal ranges. Significant differences are marked with *P<0.05, **P<0.005 and are compared to the according genotype without the TACI-Ig. (c) Mice of the indicated genotypes were observed for up to 1 year. Significant differences between genotypes are marked with *P<0.05 and were analyzed using the Logrank (Mantel–Cox) test
TACI-Ig expression ameliorates pathology in Vav-Bcl2 mice. (a) H&E staining of spleen and liver sections from 12-month-old mice killed at the end of the experiment. Magnification: 40- to 100-fold. (b) Paraffin-embedded kidney sections from mice of the indicated genotypes were taken after 12 months of phenotypically healthy mice and stained with an anti-mouse IgG antibody. # indicates specimen ID number
Discussion
BAFF was suggested to promote survival by the activation of non-canonical NF-κB signaling as well as activation of AKT/PI3K and ERK kinase modules, culminating in increased expression of Bcl2-homologs and/or the reduction of Bim levels.18, 19, 20, 29 Upon BAFF depletion, triggering Bcl2-inhibitable apoptosis (Figure 1), we noted only minor changes in Bcl2 family mRNA and protein levels (Figure 2a). Of note, pro-apoptotic Bmf and Bad were increased in MZ B cells upon BAFF depletion. Loss of either gene affects B-cell homeostasis, exacerbated in compound mutant mice,37, 38, 40 and for both proteins, a role for AKT as negative regulator has been described.47, 48 Although this fits the overall idea that loss of BAFF signaling triggers B-cell death by activating BH3-only proteins, Bim levels were found to be reduced in BAFF-sensitive B-cell stages, and, contrasting expectations, also in T1 B cells (Figure 2b). The latter observation may be explained by the fact that BAFF-R is already upregulated in transitional T1 B cells and thus aids the development of T1 B cells in the spleen.49 This suggests that only the few T1 B cells with sufficiently low levels of Bim are able to survive and progress in development in TACI-Ig mice. Reduced levels of Bcl2 and Mcl1, as noted in FO B cells, may also sensitize to apoptosis, as both proteins are clearly critical for B-cell survival.23, 50
It deserves mentioning, however, that TACI-Ig mice do not represent an exact phenocopy of Baff−/− mice,7 as BAFF may not be cleared sufficiently fast in the microenvironment where it is produced. In addition, the Fc-portion of the TACI-Ig fusion protein may trigger signaling via Fc-receptor (FcR)-activation on myeloid cells or clearance of FcR-positive B-cell subsets by alternative mechanisms than death upon BAFF depletion. Although the latter was formally excluded (Supplementary Figure 1H), the analysis of Baff−/− mice may have yielded a slightly different picture regarding the impact of BAFF depletion on Bcl2 family expression levels. However, overall our findings strongly suggest that the relative expression levels of Bcl2 proteins are rather poor predictors of biological outcome as Bim deficiency clearly protects B cells from death in TACI-Ig mice and Bmf deficiency, albeit yielding modest effects when deleted alone, strongly contributes to B-cell survival upon BAFF or APRIL depletion (Figures 3 and 4 and Supplementary Tables 1–3). Although a contribution of Bim to cell killing upon BAFF or APRIL deprivation was anticipated,31, 33 engagement of Bmf in B-cell death was unexpected, at least based on previous analysis of mature B-cell survival in culture.38 BAFF-dependent ERK activation can affect Bim stability by phosphorylation, promoting its proteasomal turnover, thereby counteracting BCR-ligation-triggered apoptosis in WEHI-231 cells.29 However, as lack of Bim conferred only partial BAFF independence to cultured B cells in our hands, additional pro-apoptotic targets neutralized by BAFF-R signaling had to be considered (Figure 3). Bmf seemed a possible candidate, as its mRNA appeared increased in T2 and FO B cells in our RT-MLPA analysis. In addition, Bmf protein was increased in MZ B cells from TACI-Ig mice (Figure 2) and FO B cells without Bmf showed reduced BAFF responsiveness in vitro (Figure 3 and Supplementary Figure 4). Notably here, growth factor deprivation or PI3K inhibition increases Bmf protein levels in different cell types, including WEHI-231 B cells47, 51 (AV unpublished). Although BAFF-dependent AKT activation appears to control mainly the metabolic fitness of B cells,21 it remains plausible that it does so, in part, by repressing Bmf. Consistently, increased cell death resistance caused by Bim/Bmf double deficiency was associated with a complete rescue of B-cell development in TACI-Ig mice (Figure 4).
Importantly, DKO mice expressing TACI-Ig were perfectly able to mount a T cell-dependent and T-independent immune response, including class switching, proving that the surviving B cells were also functional (Figure 5 and Supplementary Figure 5). These findings demonstrate that BAFF is most critical for survival, but dispensable for B-cell differentiation and activation. Further, we conclude that Bim and Bmf co-regulate B-cell homeostasis and development by acting as sensors for the presence or absence of BAFF and/or BCR signaling strength, removing cells when these signals drop under or exceed a critical threshold, respectively. As a consequence thereof, potentially autoreactive or hyperactive B cells are cleared from the system, a conclusion consistent with the noted autoimmunity in aged Bim−/−Bmf−/− mice.52 These findings define also a possible pathogenic mechanism by which B cells are depleted in CVID patients.
Remarkably, we also noted that, albeit Bcl2 overexpression induced B-cell accumulation exceeding in number that observed in DKO mice, BAFF depletion triggered a significant reduction of B cells in Vav-Bcl2/TACI-Ig mice as compared with Vav-Bcl2 mice (Figure 4 and Supplementary Table 2). This appeared paradox, as these cells do no longer respond to BAFF ex vivo and were highly apoptosis resistant (Figures 1 and 3). However, along the line of current thinking this may be explained by excessive accumulation of pro-death molecules, such as Bim,44 that are then released in the absence of BAFF, by activation of other BH3-only proteins, possibly Bmf and Bad. In accordance, Bim−/−Bmf−/− B cells were highly resistant to ABT-737, a BH3-mimetic performing similar functions as Bad,45 whereas wt and Bcl2 transgenic B cells were equally sensitive (Supplementary Figure 6). This prompted us to investigate if BAFF ablation can ameliorate the B-cell-driven pathologies in Vav-Bcl2 mice.43 Indeed, the overall survival rate of double-transgenic animals was significantly increased over that of Vav-Bcl2 mice (Figures 6 and 7). This supports the concept that BAFF-depletion therapies, alone, or in combination with Bcl2 antagonists, for example, BH3-mimetics, may prove exquisitely potent to treat B-cell-driven pathologies that associate with high levels of Bcl2, whereas the inhibition of BH3-only protein activation, when properly managed, may ameliorate B-cell loss associated with CVID.
Materials and Methods
Mouse strains
C57BL/6 TACI-Ig transgenic, Vav-Bcl2 transgenic, Bim−/−, Bmf−/−, Bad−/−, Puma−/− and Noxa−/− mice have been described.8, 33, 38, 40, 46, 53 Animal experiments were performed in agreement with Austrian legislation (BMWF-66.011/0165-II/3b/2010 and BMWF-66.011/0009-II/3b/2010).
Cell culture
Primary lymphocytes were cultured in DMEM (PAA, Linz, Austria), 250μM L-glutamine (Gibco Life-Technologies, Vienna, Austria), 50 μM 2-mercaptoethanol, penicillin/streptomycin (Sigma-Aldrich, Vienna, Austria) and 10% fetal calf serum (PAA). mBAFF (R&D Systems, Vienna, Austria) was used at 10 ng/ml. ABT-737 was purchased from Selleckchem (Houston, TX, USA).
Flow cytometry
Single-cell suspensions were stained with monoclonal antibodies conjugated with fluorescein isothiocyanate, R-phycoerythrin, allophycocyanin or biotin. The monoclonal antibodies used are listed in the Supplementary Information. Samples were analyzed in a FACS-Calibur or sorted using a FACS-AriaIII (both BD, Vienna, Austria).
Immunoblotting
Cells were lysed in RIPA buffer (150 nM NaCl, 50 mM Tris, 1% (v/v) NP40, 0.5% (v/v) sodium deoxycholate), 0.1% (v/v) SDS) with phosphatase inhibitors (PhosSTOP, Roche, Basel, Switzerland). 50 μg of protein was separated by SDS-PAGE on 10 or 14% Tris-Glycine gels and electro-blotted onto nitrocellulose membranes (Hybond, Amersham, GE Healthcare, Little Chalfont, UK). Antibodies used are listed in the Supplementary Information.
Hematopoietic reconstitution
Co-isogenic recipients were created by crossing wt Ly5.1 mice with Ly5.2 TACI-Ig transgenic mice. Mice were reconstituted via tail vein injection with 2 × 106 bone marrow cells 6 h after exposure to 10 Gy of γ-irradiation and analyzed 12 weeks after reconstitution.
RT-MLPA and qRT-PCR analysis
mRNA was isolated using Fast-Spin columns (ZymoResearch, Irvine, CA, USA). RT-MLPA analysis (MRC Holland, Amsterdam, Netherland, Mouse RT-MLPA kit RM002 or Human RT-MLPA kit R011-B1) was performed according to the manufacturer's recommendation. qRT-PCR analysis was performed as described.54 For further details see the Supplementary Information.
TACI-Ig depletion experiments
Mice were treated with graded doses of human TACI (amino acids 2–118)-hIgG1-Fc, mouse TACI (amino acids 2–78)-hIgG1-Fc produced in CHO cells, hIgG1 (Southern Biotech isotype control # 0151K-14) or PBS i.p. on days 0 and 4. On day 7, the mice were killed for analysis as described.41
Immunization
Six- to twelve-week-old mice were injected i.p. with NP-OVA (100 μg/mouse adsorbed to Alum, Sigma-Aldrich) or 50 μg TNP-Ficoll in 200 μl PBS (both Biosearch Technologies, Petaluma, CA, USA), to induce T cell-dependent or T cell-independent humoral immune responses, respectively. Blood was collected from the submandibular vein before immunization or on day 7 (TNP-Ficoll) or day 10 (NP-OVA) after immunization. Antigen-specific Ig-titers and anti-dsDNA Ig-titers were quantified by ELISA.38
Statistical analysis
Statistical analysis was performed using unpaired Student’s t-test or analysis of variance analysis, where indicated, and applying the Stat-view 4.1 software program. To compare survival of mice of different genotypes, the Log-Rank (Mantel–Cox) test was used. P values of <0.05 were considered statistically significant and marked with *, P values <0.005 are tagged with **.
Abbreviations
- BAFF:
-
B-cell activating factor of the TNF family
- TACI:
-
Transmembrane activator and CAML interactor
- BCMA:
-
B-cell maturation
- Bad:
-
Bcl2 antagonist of cell death
- Bcl-X:
-
Bcl2-related protein X
- Bcl2:
-
B-cell lymphoma 2
- BH:
-
Bcl2 homology domain
- Bim:
-
Bcl2 interacting mediator of cell death
- PUMA:
-
p53-upregulated modulator of apoptosis
- ERK:
-
extracellular-signal regulated kinase
- AKT:
-
v-AKT murine thymoma viral oncogene homolog 1
- BCR:
-
B-cell receptor
- FO:
-
follicular
- MZ:
-
marginal zone
- CVID:
-
common variable immune deficiency
- SLE:
-
systemic lupus erythematosus
References
von Boehmer H, Melchers F . Checkpoints in lymphocyte development and autoimmune disease. Nat Immunol 2010; 11: 14–20.
Herzog S, Jumaa H . Self-recognition and clonal selection: autoreactivity drives the generation of B cells. Curr Opin Immunol 2012; 24: 166–172.
Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML . B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev 2010; 237: 205–225.
Allman D, Pillai S . Peripheral B cell subsets. Curr Opin Immunol 2008; 20: 149–157.
Cerutti A, Cols M, Puga I . Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol 2013; 13: 118–132.
Kulathu Y, Grothe G, Reth M . Autoinhibition and adapter function of Syk. Immunol Rev 2009; 232: 286–299.
Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science 2001; 16: 2111–2114.
Schneider P, Takatsuka H, Wilson A, Mackay F, Tardivel A, Lens S et al. Maturation of marginal zone and follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. J Exp Med 2001; 194: 1691–1697.
Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt-Supprian M . TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J Immunol 2004; 173: 2245–2252.
Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science 2001; 293: 2108–2111.
Mackay F, Schneider P . Cracking the BAFF code. Nat Rev Immunol 2009; 9: 491–502.
Varfolomeev E, Kischkel F, Martin F, Seshasayee D, Wang H, Lawrence D et al. APRIL-deficient mice have normal immune system development. Mol Cell Biol 2004; 24: 997–1006.
Belnoue E, Pihlgren M, McGaha TL, Tougne C, Rochat AF, Bossen C et al. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood 2008; 111: 2755–2764.
Yan M, Brady JR, Chan B, Lee WP, Hsu B, Harless S et al. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B-cell deficiency. Curr Biol 2001; 11: 1547–1552.
Salzer U, Chapel HM, Webster AD, Pan-Hammarstrom Q, Schmitt-Graeff A, Schlesier M et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet 2005; 37: 820–828.
Warnatz K, Salzer U, Rizzi M, Fischer B, Gutenberger S, Bohm J et al. B-cell activating factor receptor deficiency is associated with an adult-onset antibody deficiency syndrome in humans. Proc Natl Acad Sci USA 2009; 106: 13945–13950.
Vincent FB, Saulep-Easton D, Figgett WA, Fairfax KA, Mackay F . The BAFF/APRIL system: Emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev 2013; 24: 203–215.
Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med 1999; 190: 1697–1710.
Do RK, Hatada E, Lee H, Tourigny MR, Hilbert D, Chen-Kiang S . Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J Exp Med 2000; 192: 953–964.
Hsu BL, Harless SM, Lindsley RC, Hilbert DM, Cancro MP . Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. J Immunol 2002; 168: 5993–5996.
Woodland RT, Fox CJ, Schmidt MR, Hammerman PS, Opferman JT, Korsmeyer SJ et al. Multiple signaling pathways promote B lymphocyte stimulator dependent B-cell growth and survival. Blood 2008; 111: 750–760.
Otipoby KL, Sasaki Y, Schmidt-Supprian M, Patke A, Gareus R, Pasparakis M et al. BAFF activates Akt and Erk through BAFF-R in an IKK1-dependent manner in primary mouse B cells. Proc Natl Acad Sci USA 2008; 105: 12435–12438.
Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ . Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 1993; 75: 229–240.
Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K et al. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science 2005; 307: 1101–1104.
Ottina E, Grespi F, Tischner D, Soratroi C, Geley S, Ploner A et al. Targeting antiapoptotic A1/Bfl-1 by in vivo RNAi reveals multiple roles in leukocyte development in mice. Blood 2012; 119: 6032–6042.
Tischner D, Woess C, Ottina E, Villunger A . Bcl-2-regulated cell death signalling in the prevention of autoimmunity. Cell Death Dis 2010; 1: e48.
Amanna IJ, Dingwall JP, Hayes CE . Enforced bcl-xL gene expression restored splenic B lymphocyte development in BAFF-R mutant mice. J Immunol 2003; 170: 4593–4600.
Peperzak V, Vikstrom I, Walker J, Glaser SP, LePage M, Coquery CM et al. Mcl-1 is essential for the survival of plasma cells. Nat Immunol 2013; 14: 290–297.
Craxton A, Draves KE, Gruppi A, Clark EA . BAFF regulates B cell survival by downregulating the BH3-only family member Bim via the ERK pathway. J Exp Med 2005; 202: 1363–1374.
Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity 2004; 20: 441–453.
Oliver PM, Vass T, Kappler J, Marrack P . Loss of the proapoptotic protein, Bim, breaks B cell anergy. J Exp Med 2006; 203: 731–741.
Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A . Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J Exp Med 2003; 198: 1119–1126.
Bouillet P, Metcalf D, Huang DCS, Tarlinton DM, Kay TWH, Köntgen F et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 1999; 286: 1735–1738.
Pinon JD, Labi V, Egle A, Villunger A . Bim and Bmf in tissue homeostasis and malignant disease. Oncogene 2008; 27: S41–S52.
Eldering E, Spek CA, Aberson HL, Grummels A, Derks IA, de Vos AF et al. Expression profiling via novel multiplex assay allows rapid assessment of gene regulation in defined signalling pathways. Nucleic Acids Res 2003; 31: e153.
Czabotar PE, Lessene G, Strasser A, Adams JM . Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 2013; 15: 49–63.
Baumgartner F, Woess C, Pedit V, Tzankov A, Labi V, Villunger A . Minor cell-death defects but reduced tumor latency in mice lacking the BH3-only proteins Bad and Bmf. Oncogene 2013; 32: 621–630.
Labi V, Erlacher M, Kiessling S, Manzl C, Frenzel A, O'Reilly L et al. Loss of the BH3-only protein Bmf impairs B cell homeostasis and accelerates gamma irradiation-induced thymic lymphoma development. J Exp Med 2008; 205: 641–655.
Erlacher M, Labi V, Manzl C, Bock G, Tzankov A, Hacker G et al. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med 2006; 203: 2939–2951.
Ranger AM, Zha J, Harada H, Datta SR, Danial NN, Gilmore AP et al. Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad Sci USA 2003; 100: 9324–9329.
Pelletier M, Thompson JS, Qian F, Bixler SA, Gong DH, Cachero T et al. Comparison of soluble decoy IgG fusion proteins of BAFF-R and BCMA as antagonists for BAFF. J Biol Chem 2003; 278: 33127–33133.
Ng LG, Ng CH, Woehl B, Sutherland AP, Huo J, Xu S et al. BAFF costimulation of Toll-like receptor-activated B-1 cells. Eur J Immunol 2006; 36: 1837–1846.
Egle A, Harris AW, Bath ML, O'Reilly L, Cory S . VavP-Bcl2 transgenic mice develop follicular lymphoma preceded by germinal center hyperplasia. Blood 2004; 103: 2276–2283.
Merino D, Khaw SL, Glaser SP, Anderson DJ, Belmont LD, Wong CH et al. Bcl-2, Bcl-x(L), and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood 2012; 119: 5807–5816.
Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005; 435: 677–681.
Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM . Constitutive bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci USA 1999; 96: 14943–14948.
Grespi F, Soratroi C, Krumschnabel G, Sohm B, Ploner C, Geley S et al. BH3-only protein Bmf mediates apoptosis upon inhibition of CAP-dependent protein synthesis. Cell Death Differ 2010; 17: 1672–1683.
Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997; 91: 231–241.
Rowland SL, Leahy KF, Halverson R, Torres RM, Pelanda R . BAFF receptor signaling aids the differentiation of immature B cells into transitional B cells following tonic BCR signaling. J Immunol 2010; 185: 4570–4581.
Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ . Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 2003; 426: 671–676.
Shortt J, Martin BP, Newbold A, Hannan KM, Devlin JR, Baker AJ et al. Combined inhibition of PI3K-related DNA damage response kinases and mTORC1 induces apoptosis in MYC-driven B-cell lymphomas. Blood 2013; 121: 2964–2974.
Labi V, Woess C, Tuzlak S, Erlacher M, Bouillet P, Strasser A et al. Deregulated cell death and lymphocyte homeostasis cause premature lethality in mice lacking the BH3-only proteins Bim and Bmf. Blood 2014; 123: 2652–2662.
Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 2003; 302: 1036–1038.
Tischner D, Wiegers GJ, Fiegl H, Drach M, Villunger A . Mutual antagonism of TGF-beta and Interleukin-2 in cell survival and lineage commitment of induced regulatory T cells. Cell Death Differ 2012; 19: 1277–1287.
Acknowledgements
We are grateful to K Rossi, C Soratroi, R Pfeilschifter and I Gaggl for technical assistance and/or animal care. We thank G Böck for cell sorting, M Drach for histological assessment, A Strasser and J Adams for mice and reagents as well as M Erlacher, MS Supprian and S Herzog for helpful discussion. This work was supported by grants from the Austrian Science Fund (FWF), Grants P23510-B19 and the MCBO Doctoral College Molecular Cell Biology and Oncology (W1101); the Tyrolean Science Fund (TWF) and ‘Österreichische Krebshilfe—Tirol’. CW was in part supported by a Doc-fFORTE fellowship from the Austrian Academy of Science (ÖAW). ST is supported by a Doc-Fellowship from the ÖAW. PS is supported by grants of the Swiss National Science Foundation.
Author Contributions
CW performed experiments, analyzed data, contributed to writing and prepared figures; ST and VL performed experiments, analyzed data; DB established RT-MLPA analysis; PS contributed valuable reagents and edited manuscript; AV designed research, analyzed data, wrote paper and conceived study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
PS has a research agreement with Merck-Serono. The remaining authors declare no conflict of interest.
Additional information
Edited by JP Medema
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Woess, C., Tuzlak, S., Labi, V. et al. Combined loss of the BH3-only proteins Bim and Bmf restores B-cell development and function in TACI-Ig transgenic mice. Cell Death Differ 22, 1477–1488 (2015). https://doi.org/10.1038/cdd.2015.8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2015.8
This article is cited by
-
Dynein light chain binding determines complex formation and posttranslational stability of the Bcl-2 family members Bmf and Bim
Cell Death & Differentiation (2020)
-
CHK1 dosage in germinal center B cells controls humoral immunity
Cell Death & Differentiation (2019)
-
PI3Kδ inhibition elicits anti-leukemic effects through Bim-dependent apoptosis
Leukemia (2017)
-
The BH3-only protein BIM contributes to late-stage involution in the mouse mammary gland
Cell Death & Differentiation (2016)