Abstract
c-Myc is a key transcriptional factor that has a prominent role in cell growth, differentiation and tumor development. Its protein levels are tightly controlled by ubiquitin-proteasome pathway and frequently deregulated in various cancers. Here, we report that the 11S proteasomal activator REGγ is a novel regulator of c-Myc abundance in cells. We showed that overexpression of wild-type REGγ, but not inactive mutants including N151Y and G250S, significantly promoted the degradation of c-Myc. Depletion of REGγ markedly increased the protein stability of c-Myc. REGγ interacts with the C-terminal region of c-Myc and regulates c-Myc protein turnover. Functionally, REGγ negatively regulates c-Myc-mediated cell proliferation. Interestingly, depletion of the Drosophila Reg homolog (dReg) in developing wings induced the upregulation of Drosophila Myc, which contributes to cell death. Collectively, these results suggest that REGγ proteasome has a conserved role in the regulation of Myc abundance in both mammalian cells and Drosophila.
Similar content being viewed by others
Main
Proteasome-mediated protein degradation is a central pathway that controls the function of numerous key proteins in various cellular processes.1, 2 An enzymatically active proteasome is composed of a 20S core and at least one proteasomal activator complex.3 The 20S is the catalytic core that is activated through association with proteasomal activators.4 Three proteasomal activators have been identified: 19S, PA200 and REG.4, 5 The association of 20S with 19S forms the 26S proteasome, which is responsible for targeting most of the polyubiquitinated proteins for degradation in an ATP-dependent manner.3 The 20S proteasome can also be activated by association with the REG (also known as 11S or PA28). REG-activated 20S targets proteins for degradation in an ATP- and ubiquitin-independent manner.6 Three evolutionarily conserved members of REG have been identified: REGα, REGβ and REGγ (alternatively named as PSME3 and Ki antigen).5 REGα and REGβ are mainly localized in the cytoplasm and form heteroheptamers, which are mainly involved in MHC-1-mediated immune responses in immune cells.6 In contrast, REGγ is predominantly localized within the nucleus and associated with 20S proteasome as a homoheptamer.6
Recent studies indicate that REGγ can target intact proteins for degradation in an ubiquitin- and ATP-independent manner.6 Several essential oncoproteins, including SRC-3 and PTTG1, as well as tumor suppressor proteins, such as p21 and p53, have been identified as REGγ targets.7, 8, 9, 10 REGγ is involved in the regulation of cell growth, cell cycle and apoptosis.6 REGγ is involved in the regulation of aging via p53 and regulates lipid metabolism via Sirt1 degradation.11 REGγ also affects angiogenesis by regulating PKA activity.12 REGγ is conserved in Drosophila and one Drosophila Reg (identified as dReg) has been identified.13 However, the biological function of dReg is largely unknown.
The c-Myc transcription factor is a key regulator that stimulates cell proliferation, apoptosis and differentiation.14, 15, 16, 17 Deregulated expression of c-Myc has been observed in a wide variety of human cancers.18 c-Myc is a highly unstable protein and is usually degraded in <30 min in cells.19, 20 Deregulated reduction of c-Myc protein degradation results in the accumulation of c-Myc in many cancers that may contribute to uncontrolled cell proliferation.21 However, most reported pathways that control c-Myc protein degradation are ubiquitin-dependent proteasome pathways.20 Several E3 ligases, including FBW7, SKP2, TRUSS, HectH9, β-Trcp1 and Hsc70-interacting protein (CHIP), have been identified as responsible for c-Myc degradation.19, 22, 23, 24, 25, 26, 27, 28 However, it is still unclear whether c-Myc stability is regulated by ubiquitin-independent pathways. In the present study, we demonstrated that proteasome activator REGγ is a novel negative regulator of c-Myc and provided evidence that this regulation is evolutionarily conserved.1
Results
REGγ degrades c-Myc
Previous evidence indicates that c-Myc protein levels are tightly controlled by multiple E3 ubiquitin ligases through ubiquitin-dependent protein degradation pathways under various cellular contexts.19, 26 PSMD2, a key component of 19S lid, is required for ubiquitin-dependent protein degradation by the 26S proteasome. Knockdown of PSMD2 has been shown to be an approach to inhibit the 26S proteasome activity by inhibition of 19S function.8, 29 To validate the role of the 26S proteasome in c-Myc degradation, we blocked the activity of the 26S proteasome in HeLa cells by inhibiting PSMD2 expression using PSMD2-specific siRNA as described previously.8 The turnover of endogenous c-Myc protein was examined using a cycloheximide (CHX) chase assay. Surprisingly, we found that the inhibition of PSMD2 could not completely block the protein degradation of endogenous c-Myc in HeLa cells (Figure 1a). By contrast, the turnover of p53 and p27 proteins was markedly inhibited when PSMD2 was depleted (Figure 1a). These data suggest that a 26S proteasome-independent pathway may exist to promote c-Myc degradation.
Degradation of c-Myc by REGγ. (a) HeLa cells were transfected with siRNA against PSMD2 subunit of the 19S proteasome for 72 h. Cells were treated with CHX for indicated times. The expression of c-Myc, p27, p53 and actin were determined by western blotting. (b) The 293T cells were co-transfected with hemagglutinin (HA)-c-Myc with increasing amounts of REGγ as indicated. The c-Myc protein levels were determined by western blotting. A constant amount of an enhanced green fluorescent protein (EGFP) expression plasmid was included to monitor transfection efficiency. (c) Ectopic expression of WT, but not N151Y mutant REGγ, reduced the protein levels of endogenous c-Myc. (d) Treatment with MG132 blocked the REGγ-mediated c-Myc degradation. Transfected 293T cells were treated with MG132 (20 μM) as indicated for 6 h before harvest, and cell lysates were subjected to western blotting. (e) Degradation of c-Myc by REGγ was measured by the LPDS. The luciferase activity was measured by Luciferase Reporter System (Promega). The protein abundance was determined by the ratio of FLuc/RLuc. Data were presented as means±S.D. (Student’s t-test). The actual P-values were examined using paired t-test. (f) The 293T cells were co-transfected with HA-c-Myc, REGγ, REGα, REGβ and REGα/β as indicated. The c-Myc protein levels were determined by western blotting. (g) The 293T cells were transfected as indicated. The cell extracts were examined by western blotting analysis. c-Myc was detected using an anti-HA antibody. REGγ was detected using an anti-REGγ antibody. (h and i) Effects of siRNA against REGγ on c-Myc protein levels. HeLa, MCF7 or 293T cells were transfected with two individual siRNAs (nos. 1 and 2) against REGγ. c-Myc protein levels were determined using western blotting. HeLa cells were stably expressing the short hairpin RNA (shRNA) of REGγ and the expression of c-Myc and p21 was detected using an anti-c-Myc and p21 antibody, respectively. (j) HeLa cells were transfected with 3′-UTR siRNA against REGγ and then the ectopic expression of the REGγ and REGγN151Y CDS on c-Myc protein levels were determined using western blotting. (k) Effect of RNAi of REGγ on c-Myc half-life. HeLa cells were transfected with siRNA against REGγ. After 72 h, cells were treated with CHX for the indicated time. Cells were lysed, and cell lysates were subjected to western blotting analysis. The c-Myc level at each time point is represented relative to the level at time zero
In addition to 19S lid, proteasome activator REG can form an 11S regulatory cap to activate the 20S proteasome.6 REGγ is emerging as an alternative pathway to target intact proteins for degradation independent of the 26S proteasome.7, 30 Thus, we examined whether REGγ has any role in c-Myc protein turnover. Our data showed that ectopic expression of REGγ resulted in a marked reduction in c-Myc protein in a dose-dependent manner (Figure 1b). Ectopic expression of wild-type (WT), but not inactive mutant N151Y REGγ also promoted the degradation of endogenous c-Myc in HeLa cells (Figure 1c). Furthermore, treatment with MG132 (carbobenzoxy-L-leucyl-L-leucyl-L-leucinal), a potent proteasome inhibitor, completely blocked the REGγ-mediated c-Myc degradation (Figure 1d), indicating that REGγ-promoted c-Myc degradation is dependent on the proteasome activity.
To further validate whether REGγ promotes c-Myc degradation independently of transcription, we set up a luciferase-based protein degradation (LPDS) reporter system (Supplementary Figure S1A). The LPDS vector expresses a single transcript encoding firefly luciferase (FLuc) and Renilla luciferase (RLuc) separated by an internal ribosome entry site. Both FLuc and RLuc are quite stable and not rapidly destructed by the proteasome degradation pathway (Supplementary Figure S1B). When fused with a short-lived protein to C terminus, the abundance of FLuc protein is controlled by the stability of the fused protein. In contrast, RLuc protein was not affected by the fusion because it is translated independent of FLuc. Therefore, the FLuc/RLuc ratio can reflect the turnover rate of the fused protein. Taking advantage of this system, we validated the role of REGγ in c-Myc degradation. Our data showed that REGγ had little effect on the FLuc/RLuc ratio when coexpressed with vector without fusion to any other protein (Supplementary Figure S1B). In contrast, when fused with c-Myc to the C terminus of FLuc, the FLuc/RLuc ratio was markedly decreased by the coexpression of REGγ (Figure 1e). Importantly, treatment with MG132 completely inhibited the effect of REGγ (Figure 1e). This confirms that REGγ can indeed promote c-Myc degradation.
To further illustrate the specificity of REGγ, we evaluated the degradation of c-Myc by overexpressing c-Myc with REGα, REGβ, REGγ and REGα/β in 293T cells. As shown in Figure 1f, REGγ, but not REGα and REGβ, degraded c-Myc. As a proteasome activator, REGγ must associate with the 20S proteasome to promote protein degradation.6 Mutation of Asn151 to Tyr (N151Y) or Gly150 to Ser (G150S) impairs the ability of REGγ to activate the 20S proteasome, but does not affect its binding to 20S proteasomes.31 K188D is a hyperactive mutant that can activate trypsin-like, chymotrypsin-like and postglutamyl peptidyl hydrolyzing activity of the 20S proteasome.32 As shown in Figure 1g, WT and hyperactive K188D, but not N151Y and G150S REGγ, markedly promoted c-Myc degradation. These results suggest that degradation of c-Myc by REGγ requires the activation of the 20S proteasome.
Endogenous c-Myc is regulated by REGγ in cancer cells
Next, we determined whether endogenous c-Myc is regulated by endogenous REGγ. To this end, REGγ was depleted using two independent siRNAs against REGγ in HeLa cells for 72 h. Western blotting analysis showed that endogenous cellular REGγ was efficiently depleted by both siRNAs. We found that endogenous c-Myc protein was markedly increased in REGγ-depleted cells (Figure 1h). Stable knockdown of REGγ in HeLa cells also markedly increased the protein levels of both c-Myc and p21 (Figure 1h). Similar results were obtained in MCF7 and 293T cells (Figure 1i). Moreover, expression of an RNA interference (RNAi)-resistant WT REGγ, but not the inactive REGγN151Y, decreased the c-Myc protein (Figure 1j), indicating that c-Myc protein stability was specifically regulated by REGγ expression and activity.
The effect of endogenous REGγ on the degradation of endogenous c-Myc was determined using a CHX chase assay. As shown in Figure 1k, the half-life of endogenous c-Myc protein was markedly prolonged upon REGγ knockdown. Moreover, knockdown of REGγ or FBW7 strikingly increased c-Myc stability in the LPDS assay (Supplementary Figure S1C), confirming that c-Myc stability was regulated by endogenous REGγ.
Phosphorylation of c-Myc at Thr58 or Ser62 has been shown to be important for the ubiquitin-dependent degradation of c-Myc by FBW7.19 However, our data indicated that T58A/S62A was still degraded efficiently by REGγ (Supplementary Figure S1D), suggesting that degradation of c-Myc by REGγ is independent of FBW7.
REGγ deficiency stabilizes c-Myc in MEFs
We further examined the regulation of c-Myc by REGγ using REGγ−/− (REGγ knockout) murine embryonic fibroblasts (MEFs). As expected, REGγ deficiency significantly increased the protein levels of c-Myc as well as the reported REGγ substrates p53 and p21,10, 30 but had no measurable effect on the protein levels of AKT and LSD1 (Figure 2a). This result was confirmed using an immunofluorescence staining assay (Figure 2b). REGγ deficiency markedly increased c-Myc protein levels in the nuclei of MEFs (Figure 2b). Moreover, our data showed that the half-life of c-Myc was markedly increased in REGγ−/− MEFs than in WT MEFs (Figure 2c). We also assessed the mRNA level of c-Myc in REGγ−/− MEFs. Surprisingly, we found that c-Myc mRNA level was also slightly increased in REGγ−/− MEFs compared with WT MEFs (Supplementary Figure S2), suggesting that REGγ may also regulate c-Myc at the transcriptional level. The ectopic expression of exogenous REGγ, but not the inactive mutant REGγN151Y in REGγ−/− MEFs, decreased the half-life of c-Myc protein (Figure 2d). These data indicate that endogenous c-Myc is a target of REGγ in MEFs.
Regulation of c-Myc by REGγ in MEFs. (a) The protein levels of c-Myc, p21, p53, AKT, and LSD1 were determined in REGγ +/+ and REGγ −/− MEFs. (b) The expression levels of c-Myc in MEFs were examined using immunofluorescence. (c) MEFs were treated with CHX for the indicated time. c-Myc was detected using western blotting. Quantification of c-Myc levels is shown. (d) REGγ−/− MEFs were infected with lentivirus-expressing vector, REGγ or REGγ N151Y for 72 h. Cells were treated with CHX for the indicated time and half-lives of c-Myc were determined using western blotting. Quantification of the c-Myc levels is shown. The c-Myc level at each time point is represented relative to the level at time zero
c-Myc associates with REGγ
Physical interaction between c-Myc and REGγ was determined using a co-IP assay. Our data showed that exogenous REGγ could interact with c-Myc in cells (Figure 3a). Mutation of c-Myc Thr58 or Ser62 had no effect on its interaction with REGγ (Supplementary Figure S3A). We also examined the interaction between endogenous c-Myc and REGγ. HeLa cells were treated with MG132 and endogenous c-Myc was immunoprecipitated using an anti-c-Myc antibody. We found that REGγ could be readily detected in c-Myc immunoprecipitates (Figure 3b). Endogenous c-Myc was also present in the endogenous REGγ immunoprecipitates (Figure 3c). These data indicate that c-Myc can associate with REGγ in cells.
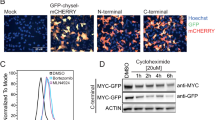
Interaction between REGγ and c-Myc. (a) GFP-REGγ and hemagglutinin (HA)-c-Myc were transfected into 293T cells. Cells were treated with MG132 and cell lysates were immunoprecipitated using an anti-HA antibody. The interaction was analyzed using western blotting. (b and c) Interaction between endogenous c-Myc and REGγ. HeLa cells were treated with MG132 for 6 h. c-Myc was immunoprecipitated with an anti-c-Myc or REGγ antibody or irrelevant immune serum. The interaction was analyzed using western blotting. (d) REGγ interacts with the C terminus of c-Myc. The structural domains of c-Myc are shown. (e) Identification of domains in REGγ required for its interaction with c-Myc. The structural domains of REGγ are shown. ‘−’ marks a lack of detectable interaction, whereas ‘+’ marks interaction. A, acidic domain; B, basic; HLH, helix–loop–helix; LZ, leucine zipper; MB, Myc boxes; NLS, nuclear localization sequence; WCE, whole-cell extract
To define the domains in c-Myc that are responsible for its interaction with REGγ, we generated a series of c-Myc deletion mutants. Our data showed that REGγ interacted with those mutants containing the C-terminal domain including c-Myc (1–439 aa), c-Myc (143–439 aa) and c-Myc (251–439 aa). Thus, we conclude that the C-terminal domain of c-Myc is responsible for its interaction with REGγ (Figure 3d). The recent study indicated that stress can induce the production of an Myc-nick (short form of c-Myc), which promoted cell survival and autophagy.33, 34 Our data indeed showed that Myc-nick was easily detected when cells were cultured at dense condition (Supplementary Figure S3C). However, Myc-nick cannot bind to (Supplementary Figure S3B) and be degraded by REGγ (Supplementary Figure S3C). We also mapped the domains of REGγ involved in its interaction with c-Myc using a co-IP assay and in vitro pull-down assay. Our data indicated that both the N- and C-terminal regions of REGγ could interact directly with c-Myc, although with a weaker affinity than WT REGγ. (Figure 3e and Supplementary Figure S3D). To examine whether multimer of REGγ is required for its binding to c-Myc, we coexpressed a mutant of REGγ K195R, which cannot form the multimer in cells.35 Our data indicated that K195R mutant could still bind to c-Myc, but cannot degrade c-Myc anymore (Supplementary Figures S3E and F). Collectively, these data indicate that c-Myc can physically interact with REGγ.
REGγ regulates c-Myc-mediated gene expression
c-Myc is a transcription factor that may regulate the transcription of numerous genes.36 Because REGγ can promote the degradation of c-Myc, we examined whether REGγ affects c-Myc-mediated gene expression using a luciferase reporter gene assay. It has been shown that expression of c-Myc can activate the promoter activity of RhoA and E2F1.37, 38 Consistent with previous reports,37, 39 expression of c-Myc strikingly increased the RhoA and E2F1 promoter activity (Figure 4a). As expected, the coexpression of REGγ significantly reduced the c-Myc-activated RhoA and E2F1 promoter activity. These data indicate that ectopically expressed REGγ can inhibit c-Myc transcriptional activity.
REGγ inhibits c-Myc-induced cell proliferation. (a) Ectopic expression of REGγ inhibited c-Myc transcriptional activity. The 293T cells were transfected with pGL3-RhoA (left) or E2F1 (right) promoter reporter gene together with the c-Myc or REGγ plasmids as indicated. The promoter activity was measured 36 h after transfection. Data were presented as means±S.D. (Student’s t-test), and the actual P-values were examined using paired t-test. (b) HeLa cells were transfected with siRNA as indicated; 24 h later, cells were transfected with 10 ng Renilla plasmids together with pGL3-RhoA promoter. After 36 h, the promoter activity was measured using luciferase assay. Data were presented as means±S.D. (Student’s t-test), and the actual P-values were examined using paired t-test. (c) HeLa cells were transfected with siRNA as indicated for 72 h. The relative expression of CDK4 was measured using q-PCR. Data were presented as means±S.D. (Student’s t-test), and the actual P-values were examined using paired t-test. (d) The effect of REGγ on cell proliferation was measured using an EdU incorporation assay. Data were presented as means±S.D. (Student’s t-test), and the actual P-values were examined using paired t-test. (e) Colony formation assay was performed using HeLa cells transfected with siRNA as indicated. Data were presented as means±S.D. (Student’s t-test), and the actual P-values were examined using paired t-test. (f) HeLa cells transfected with siRNA as indicated. Cell growth was measured using an MTT assay Data were presented as means±S.D. (Student’s t-test), and the actual P-values were examined using paired t-test. (g) HeLa cells were transfected as indicated and cell growth was measured using soft-agar assay. Data were presented as means±S.D. (Student’s t-test), and the actual P-values were examined using paired t-test. RLA, relative luciferase activity
We also examined the effect of endogenous REGγ on c-Myc-mediated gene expression. Our data showed that knockdown of REGγ significantly increased the activity of RhoA promoter, which could be reversed by c-Myc knockdown (Figure 4b). Moreover, knockdown of REGγ also increased the mRNA level of CDK4, a downstream target of c-Myc (Figure 4c). Collectively, these results indicate that REGγ is a negative regulator of c-Myc-mediated gene expression.
REGγ inhibits c-Myc-induced cell proliferation
c-Myc is an essential factor that regulates cell proliferation.17 Because REGγ can regulate the protein stability of c-Myc, we asked whether REGγ affects c-Myc-mediated cell proliferation. HeLa cells were transfected with c-Myc in the presence of WT or N151Y REGγ and cell proliferation was measured using a 5-ethynyl-2′-deoxyuridine (EdU) incorporation assay. As expected, the expression of c-Myc in HeLa cells markedly promoted EdU incorporation (Figure 4d). Coexpression of WT, but not N151Y REGγ, markedly blocked the c-Myc-mediated EdU incorporation (Figure 4d).
We also examined the effect of endogenous REGγ on the proliferation of HeLa cells. To this end, HeLa cells transfected with control or REGγ siRNA were cultured for 21 days and then stained with crystal violet to count for colony numbers. As shown in Figure 4e, knockdown of REGγ resulted in a significant increase in proliferation of HeLa cells. The knockdown of c-Myc could rescue the effect of REGγ siRNA on cell proliferation (Figure 4e), suggesting that c-Myc is likely involved in REGγ-mediated cell proliferation. This result was confirmed using a MTT (3-[4,5-dimethylthiozol-2-yl]-2,5diphenyltetrazoliumbromide) assay (Figure 4f).
We confirmed the role of REGγ in c-Myc-mediated cell growth using a soft-agar colony formation assay. Knockdown of REGγ in HeLa cells markedly increased the colonies number, which could be reversed by c-Myc knockdown (Figure 4g). Taken together, these data suggest that REGγ is a negative regulator of c-Myc-mediated cell growth.
Regulation of dMyc stability by REG in Drosophila
REGγ has one Drosophila ortholog, dReg;13 however, the biological function of dReg is largely unknown. Myc is a conserved transcriptional factor and has essential function in Drosophila.40, 41 To examine whether dReg regulates Drosophila Myc (dMyc) level in Drosophila, we depleted dReg in Drosophila S2 cells and detected the dMyc protein level by western blotting. As shown in Figure 5a, depletion of dReg in S2 cells markedly increased dMyc protein levels. In addition, knockdown efficiency of dReg and dMyc transcriptional level was confirmed by q-PCR (Figure 5b). Moreover, depletion of dReg in S2 cells prolonged the half-life of dMyc protein with the treatment of CHX at various time points (Figures 5c and d).
Deletion of dReg in vitro and in vivo enhanced dMyc protein abundance. (a) S2 cells were cultured according to a standard protocol as described previously. GFP dsRNA and dReg dsRNA were generated by MEGAscript High Yield Transcription Kit (Ambion; no. AM1334). After 72 h, cell extracts were subjected to immunostaining with dMyc and tubulin antibody. (b)The knockdown efficiency of endogenous dReg and mRNA levels of dMyc were determined using RT-PCR. (c) Pulse chase for endogenous dMyc in the presence and absence of dReg. CHX were added to block cellular protein synthesis at different time points. (d) Quantification of dMyc stability to show extended dMyc half-life by dRegRNAi. The dMyc level at each time point is represented relative to the level at time zero. (e–h) Depletion of dReg promoted dMyc stabilization in wing discs. (e) MS1096-Gal4 was used to drive the expression UAS-dRegRNAi or UAS-AgoRNAi. Western blotting showed dMyc accumulation in discs with either dReg or Ago depleted. Tubulin was used as a loading control. (f) RT-q-PCR showed that dReg mRNA was downregulated specifically by dRegRNAi. (g) Immunostaining of dMyc in a late third instar control wing disc and (h) a wing disc expressing MS1096>dRegRNAi. Of note, MS1096-Gal4 drives UAS transgene expression at higher levels in the dorsal region of wing discs (arrow)
To further examine whether dMyc is regulated by dReg in vivo, we performed a loss-of-function analysis of dReg in Drosophila using the Gal4-UAS system to drive the expression of a transgenic dReg RNAi line (UAS-dRegRNAi) in a tissue-specific manner.42 We used MS1096-Gal4 to deplete dReg in the entire wing pouch regions. Depletion of dReg using MS1096-Gal4 significantly increased dMyc protein detected by both immunostaining and western blotting assays (Figures 5e, g and h). Archipelago (Ago) is the Drosophila homolog of Fbw7 and AgoRNAi was used as a positive control for dMyc stabilization. The dReg knockdown efficiency was confirmed using q-PCR (Figure 5f). These results demonstrate that dReg is a negative regulator of dMyc.
dReg promotes cell survival by preventing aberrant dMyc accumulation
Because upregulation of dMyc in Drosophila triggers cell-autonomous apoptosis,41 we asked whether loss of dReg provokes cell death via dMyc upregulation. To this end, we depleted dReg in the dorsal compartment of wing discs using apterous-Gal4 (ap-Gal4). UAS-GFP was coexpressed to mark the cells with dReg depleted. dMyc protein was uniformly distributed in the dorsal and ventral compartments in the control wing discs (Figures 6a and b). As expected, dMyc protein level was markedly increased in dorsal compartment cells where dReg was depleted (Figures 6d and e). We monitored apoptosis using an antibody against activated caspase-3 and found that dorsal compartment cells with dReg depleted contained excessive number of caspase-3-positive cells, which were barely detected in control discs (compare Figure 6c with Figure 6f).
Depletion of dReg induced ectopic apoptosis phenotype because of dMyc upregulation. (a–o) Late third instar wing discs of the indicated genotypes were immunostained with GFP (green), dMyc (red) and cleaved caspase-3 (red) antibodies. Control discs expressing UAS-GFP under the control of ap-Gal4 exhibited equal dMyc staining in dorsal versus ventral compartment and little if any caspase-3-positive cells (a–c). Knockdown of dReg in dorsal compartment cells upregulated dMyc and induced apoptosis (arrows in d–f). Simultaneous knockdown of dMyc and dReg prevented dMyc upregulation and rescued the cell death phenotype induced by dReg depletion (g–i), whereas dMyc knockdown alone did not induce apoptosis (j–l). Coexpression of the cell death inhibitor Diap1 with dRegRNAi transgene rescued the cell death phenotype but did not block dMyc upregulation induced by dReg depletion (m–o)
To determine whether elevated dMyc in dReg-depleted cells contribute to cell death, we knocked down dMyc by RNAi either in control or dReg-depleted discs. dMyc knockdown was confirmed by staining the discs with the dMyc antibody (Figures 6j and k). Although dMyc RNAi alone did not have a significant effect on cell death (Figure 6l), dMyc RNAi in dReg depletion discs prevented the upregulation of dMyc and greatly reduced the number of caspase-3-positive cells (Figures 6g–i), suggesting that dReg promotes cell survival at least in part by preventing the abnormal accumulation of dMyc. Coexpression of the cell death inhibitor Diap1 with dRegRNAi blocked apoptosis but did not block the upregulation of dMyc (Figures 6m–o), suggesting that upregulation of dMyc in dReg-depleted cells is not secondary to cell death but instead may reflect a direct regulation of dMyc by dReg. Consistent with this, we found that an HA-tagged dReg formed a complex with a Flag-tagged dMyc when coexpressed in S2 cells (Supplementary Figure S4). Taken together, our data indicate that Reg has a conserved role in regulating Myc abundance.
Loss of dReg causes developmental defects
We further used the Gal4-UAS system, which can drive the expression of a transgenic dReg RNAi line (UAS-dRegRNAi) in a tissue-specific manner, to analyze the phenotype caused by dReg loss-of-function in Drosophila. We used ap-Gal4 driver to specifically deplete dReg in the dorsal compartment of the wing (Figures 7a and b), MS1096-Gal4 to deplete dReg in the whole wing pouch regions (Figures 7c and d) and Hh-Gal4 to induce UAS-RNAi expression in the posterior part of the wing (Figures 7e and f). Our data indicated that depletion of dReg in the wing resulted in the smaller wing size compared with the control wing (Figures 7a–d). Moreover, dReg depletion by Hh-Gal4 driver had marked ablation of posterior wing phenotype (Figures 7e and f). Furthermore, loss of dReg expression in Drosophila eye elicited by eyeless-Gal4 showed smaller eye phenotype (Figures 7g and h). In summary, we demonstrate that dReg expression is involved in normal development likely by regulating apoptosis through dMyc.
Deletion of dReg displayed abnormal tissue phenotype. (a and b) Downregulation of dReg by ap-Gal4, which specifically expressed in the wing dorsal compartment, contributed to a significantly minor size wing. (c and d) Downregulation of dReg by MS1096-Gal4, which was specifically expressed in whole wing discs, contributed to a significantly minor size wing. (e and f) dReg reduction by Hh-Gal4 driver in the posterior part had marked ablation of posterior wing phenotype. (g and h) Loss of dReg expression in Drosophila eye by eyeless-Gal4 showed a significantly smaller eye. WT control was driven by using each of Gal4
Discussion
As an essential transcription factor, the protein levels of c-Myc are tightly controlled by multiple E3 ubiquitin ligases through ubiquitin-dependent pathways.20 In this study, we identified a novel ubiquitination-independent pathway regulating the protein levels of c-Myc. We found that the proteasome activator REGγ controls the abundance of c-Myc proteins by regulating protein stability. REGγ interacts with c-Myc and promotes its turnover in cells. REGγ inhibits c-Myc-mediated gene expression and cell growth. In addition, regulation of Myc stability by dReg is essential for Drosophila development. Thus, our study suggests that REGγ and dReg are novel and conserved regulators of Myc proteins.
As a component of 11S cap, it was originally recognized that REGγ only has the ability to degrade unfolded proteins or small peptides by activating the 20S proteasome.6 However, this idea has been challenged by the recent discovery that REGγ can degrade intact oncoprotein SRC-3.7 Following that finding, more proteins, such as p21, ARF, p14, MAFA, and AID, were found to be degraded by REGγ.8, 30, 43, 44 Interestingly, REGγ can degrade oncoproteins including SRC-3 and PTTG1, as well as tumor suppressors including p21, ARF and p53.7, 8, 9, 10 Thus, it is expected that REGγ has a context-dependent role in tumorigenesis. Indeed, studies indicated that REGγ is absent in breast cancers but upregulated in thyroid cancers.7, 45 Our study revealed that the oncoprotein c-Myc is a novel substrate of REGγ. We found that c-Myc protein is negatively regulated by REGγ in several cancer cell lines, including HeLa, MCF7 and MEFs. Deregulation of c-Myc protein stability has been shown to be related to tumorigenesis.17 Our data clearly showed that knockdown of REGγ promoted the cell proliferation of cancer cells via a c-Myc-dependent way, supporting the role of REGγ in the regulation of cell proliferation and tumorigenesis.
Multiple pathways have been identified to control the c-Myc protein stability, such as FBW7, SKP2, β-Trcp1 and CHIP.23, 24, 26, 27 Thus, it is likely that the regulation of c-Myc stability by various pathways is context-dependent or cell-type-specific. Indeed, our unpublished data showed that knockdown of REGγ in several cell lines, such as A549 and HCT116, had no measurable effect on c-Myc protein levels (data not shown). The mechanism of cell-context-dependent regulation of c-Myc is still unclear. It is possible that the expression levels of REGγ or other c-Myc regulators may be different in different types of cells, which may contribute to the cell-type-specific regulation of c-Myc stability by REGγ. It is also possible that REGγ-mediated c-Myc degradation is regulated by context-dependent extracellular signaling events.46 In addition, some unidentified regulators may affect the regulation of c-Myc by REGγ. Thus, more studies will be required to characterize the regulation of c-Myc by REGγ under different physiological and pathological conditions in future.
The regulation of Myc by REGγ appears to be conserved in Drosophila. We found that dReg interacted with dMyc and that dReg depletion increased the stability of dMyc in cultured cells. In addition, we found that dReg depletion in wing discs upregulated dMyc protein levels, leading to apoptosis. These results suggest that dReg promotes cell survival in Drosophila development by preventing abnormal dMyc accumulation. Our study on the regulation of Myc by REGγ in Drosophila not only demonstrated the physiological relevance but also the evolutionary conservation of this regulatory mechanism.
Materials and Methods
Plasmids
The expression plasmids of WT and N151Y REGγ were described previously.30 RhoA promoter reporter was kindly provided by Dr. Hui-Kuan Lin.37 E2F1 promoter reporter was kindly provided by Dr. Mu-Shui Dai.39 Mouse c-Myc plasmids were amplified using PCR and cloned into pcDNA3.1 with an HA tag at the N terminus. Point mutations and deletion mutants of c-Myc and REGγ were cloned using PCR-based standard cloning method. GFP-tagged REGγ deletion mutants were kindly provided by Dr. Chuangui Wang (East China Normal University, Shanghai, China).11 Lentivirus vectors were produced by subcloning REGγ and its mutants into PLVX-ZsGreen lentivirus vector. The LPDS system was produced by subcloning firefly and Renilla CDS into pcDNA3.1 vector. c-Myc was subcloned into the C terminus of FLuc. All the vectors were confirmed using DNA sequencing.
Reagents
Antibodies against p21 (no. 2136), p53 (no. 9282), LSD1 (no. 2139), AKT (no. 9272) and p27 (no. 2552) were obtained from Cell Signaling Technology (Danvers, MA, USA). c-Myc (no. 1472-1) antibody was purchased from Epitomics (Burlingame, CA, USA). The Myc 274 antibody against Myc-nick is a generous gift from Naohiko Ikegaki (University of Illinois, Champaign, IL, USA).33 Anti-actin (sc-8432), REGγ (sc133876), c-Myc (9E10) (sc-40) and PSMD2 (sc-68352) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-HA (H3663), Flag (F1840) and GFP (G1546) antibodies, CHX (C4859) and MG132 (M7449) were all purchased from Sigma (St. Louis, MO, USA). Dual-Luciferase Reporter System Kit (E1501) and TNT (L5020) were purchased from Promega (Madison, WI, USA).
Cell culture and transfections
Human embryonic kidney 293T (HEK293T) cells and human epithelial carcinoma HeLa cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) at 37 °C in 5% CO2. MEFs were isolated from E13.5-day REGγ+/+, REGγ−/− mouse embryos. Immortalized MEFs were described in a previous report.30 All cell lines were cultured in DMEM supplemented with 10% FBS (HyClone, Logan, UT, USA). Transfections were performed using the calcium phosphate-DNA co-precipitation method for HEK293T cells, and SunbioTrans-EZ for HeLa cells.
RNA interference
Three different siRNAs against REGγ were used: siRNA no. 1 – 5′-GAAUCAAUAUGUCACUCUAUU-3′ and siRNA no. 2 – 5′-UCUGAAGGAACCAAUCUUAUU-3′. siRNA no. 3: 5′-GGGAACUAUUUCUCUUUAUTT-3′ and the siRNA against c-Myc was: 5′-CAUCAUCAUCCAGGACUGUAU-3′. PSMD2 siRNAs used were as follows: no. 1: 5′-GCGACCUGCUUAU;GGAAAUTT-3′ and no. 2: 5′-CCACUAUCCUUCAGACCAUTT-3′.
CHX chase assay
The siRNAs were transfected into cells using Lipofectamine 2000 following the manufacturer’s instruction. At 72 h after transfection, cells were treated with 50 μg/ml CHX for the indicated times. Then, the cells were lysed and prepared for western blotting analysis. c-Myc levels relative to time point 0 are shown in all graphs of CHX experiments.
IP and western blotting
Cells were transfected, treated with 10 μM MG132 for 6 h and lysed using 2 × RIPA buffer (Tris-HCl, pH 7.4 (100 mM); NaCl (300 mM); 0.1% NP-40; 2% sodium deoxycholate; NaF (10 mM); and Na3VO4 (10 mM). The cell lysates were cleared by centrifugation and incubated with 1 μg indicated antibody overnight at 4 °C followed by incubation with 15 μl protein A and G beads (Santa Cruz) for 2 h at 4 °C. IPs were subjected to western blotting as described.47
EdU incorporation assay
EdU, a thymidine analog, was used to detect cell proliferation by incorporating into cellular DNA during DNA replication. Briefly, HeLa cells were transfected for 72 h and then analyzed by EdU incorporation assay according to the manufacturer’s instructions (Ribobio Cell-Light EU Apollo567 In Vitro Imaging Kit; Ribobio, Guangzhou, China).
RNA extraction and Q-PCR
Q-PCR was performed in duplicate using the SYBR Premix ExTaq (Takara, Otsu, Japan; DRR420A) on an Mx3000P System (Stratagene, Agilent Technologies, Santa Clara, CA, USA). Data were calculated according to the ΔCt relative quantification method. Primers used for RT-q-PCR were as follows: Gapdh (sense: 5′-AGCCATCACAGTCGATTC-3′; antisense: 5′-CCGATGCGACCAAATCCAT-3′), CDK4 (sense: 5′-TGGTGTCGGTGCCTATGG-3′; antisense: 5′-GAACTGTGCTGATGGGAAGG-3′) and dMyc (sense: 5′-AGCATCACCACCAACAACAA-3′, antisense: 5′-TTGACTGCGAACTGGAACTG-3′).
MTT assay
HeLa cells transfected with siRNA were performed in 96-well plates. Forty-eight hours after transfection, MTT (T0793; Sangon Biotech Shanghai Co., Ltd, Shanghai, China) was added to each well for 4 h. The reaction was stopped by adding 150 μl DMSO and the absorbance was measured at 490 nm using a microplate spectrophotometer (SpectRA MAX190; Molecular Devices Corp, Sunnyvale, CA, USA).
Colony formation assay
Soft-agar colony formation assays were carried out in six-well dishes. Briefly, HeLa cells (2.5 × 103) were transfected with either siNC, siREGγ or sic-Myc. Cells were mixed with 0.8% agarose in warm 2 × DMEM containing 20% FBS and plated in each well of a 6-well plate on top of a prepared 1.2% agar base. Colony formation was assessed by counting the number of colonies under low magnification ( × 100) at four points on each well. A colony was defined as >10 cells in one site.
Drosophila stocks
Flies were raised at 25 °C with a standard protocol. w1118 were used as WT control for all cross- experiments with various Gal4. UAS-RegRNAi (V110156) RNAi fly stock was purchased from Vienna Drosophila RNAi Center (Vienna, Austria). UAS-Diap1 (6657), UAS-dMycRNAi (TRiP25783), UAS-AgoRNAi (TRiP34802) stocks were obtained from Bloomington Stock Center (Bloomington, IN, USA).
Immunostaining
Late third instar larvae were dissected as per the protocol described previously. Antibodies that were used for immunostaining were as follows: anti-Myc (d1–717) (sc-28207) was obtained from Santa Cruz and anti-cleaved caspase-3 (9661) was purchased from Cell Signaling.
GST-pull-down assay
In vitro transcribed and translated c-Myc protein was produced using TNT T7 SP6 Coupled Reticulocyte Lysate System Kit (Promega; L5020) according to the manufacturer’s instructions. The c-Myc proteins were incubated with glutathione S-transferase (GST) or GST-REGγ (WT), GST-REGγ (1–96), (96–255) protein for 2 h at 4 °C. The GST proteins were purified using glutathione sepharose 4B (Amersham Biosciences, Amersham, UK), and the bound c-Myc was detected using western blotting.
Abbreviations
- GST:
-
glutathione S-transferase
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- CHX:
-
cycloheximide
- MG132:
-
carbobenzoxy-L-leucyl-L-leucyl-L-leucinal
- IP:
-
immunoprecipitation
- Edu:
-
5-ethynyl-2′-deoxyuridine
- MTT:
-
3-[4,5-dimethylthiozol-2-yl]-2,5diphenyltetrazoliumbromide
- MEF:
-
murine embryonic fibroblast
References
Adams J . The proteasome: structure, function, and role in the cell. Cancer Treat Rev 2003; 29 (Suppl 1): 3–9.
Moreau P, Richardson PG, Cavo M, Orlowski RZ, San Miguel JF, Palumbo A et al. Proteasome inhibitors in multiple myeloma: 10 years later. Blood 2012; 120: 947–959.
Voges D, Zwickl P, Baumeister W . The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochem 1999; 68: 1015–1068.
Baumeister W, Walz J, Zuhl F, Seemuller E . The proteasome: paradigm of a self-compartmentalizing protease. Cell 1998; 92: 367–380.
Rechsteiner M, Realini C, Ustrell V . The proteasome activator 11 S REG (PA28) and class I antigen presentation. Biochem J 2000; 345 (Part 1): 1–15.
Mao I, Liu J, Li X, Luo H . REGgamma, a proteasome activator and beyond? Cell Mol Life Sci 2008; 65: 3971–3980.
Li X, Lonard DM, Jung SY, Malovannaya A, Feng Q, Qin J et al. The SRC-3/AIB1 coactivator is degraded in a ubiquitin- and ATP-independent manner by the REGgamma proteasome. Cell 2006; 124: 381–392.
Chen X, Barton LF, Chi Y, Clurman BE, Roberts JM . Ubiquitin-independent degradation of cell-cycle inhibitors by the REGgamma proteasome. Mol Cell 2007; 26: 843–852.
Ying H, Furuya F, Zhao L, Araki O, West BL, Hanover JA et al. Aberrant accumulation of PTTG1 induced by a mutated thyroid hormone beta receptor inhibits mitotic progression. J Clin Invest 2006; 116: 2972–2984.
Zhang Z, Zhang R . Proteasome activator PA28 gamma regulates p53 by enhancing its MDM2-mediated degradation. EMBO J 2008; 27: 852–864.
Dong S, Jia C, Zhang S, Fan G, Li Y, Shan P et al. The REGgamma proteasome regulates hepatic lipid metabolism through inhibition of autophagy. Cell Metab 2013; 18: 380–391.
Liu S, Lai L, Zuo Q, Dai F, Wu L, Wang Y et al. PKA turnover by the REGgamma-proteasome modulates FoxO1 cellular activity and VEGF-induced angiogenesis. J Mol Cell Cardiol 2014; 72C: 28–38.
Masson P, Lundgren J, Young P . Drosophila proteasome regulator REGgamma: transcriptional activation by DNA replication-related factor DREF and evidence for a role in cell cycle progression. J Mol Biol 2003; 327: 1001–1012.
Adhikary S, Eilers M . Transcriptional regulation and transformation by Myc proteins. Nat Rev Mol Cell Biol 2005; 6: 635–645.
van Riggelen J, Yetil A, Felsher DW . MYC as a regulator of ribosome biogenesis and protein synthesis. Nat Rev Cancer 2010; 10: 301–309.
Eilers M, Eisenman RN . Myc’s broad reach. Genes Dev 2008; 22: 2755–2766.
Dang CV . MYC on the path to cancer. Cell 2012; 149: 22–35.
Nesbit CE, Tersak JM, Prochownik EV . MYC oncogenes and human neoplastic disease. Oncogene 1999; 18: 3004–3016.
Welcker M, Orian A, Jin J, Grim JE, Harper JW, Eisenman RN et al. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc Natl Acad Sci USA 2004; 101: 9085–9090.
Thomas LR, Tansey WP . Proteolytic control of the oncoprotein transcription factor Myc. Adv Cancer Res 2011; 110: 77–106.
Salghetti SE, Kim SY, Tansey WP . Destruction of Myc by ubiquitin-mediated proteolysis: cancer-associated and transforming mutations stabilize Myc. EMBO J 1999; 18: 717–726.
Adhikary S, Marinoni F, Hock A, Hulleman E, Popov N, Beier R et al. The ubiquitin ligase HectH9 regulates transcriptional activation by Myc and is essential for tumor cell proliferation. Cell 2005; 123: 409–421.
Yada M, Hatakeyama S, Kamura T, Nishiyama M, Tsunematsu R, Imaki H et al. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J 2004; 23: 2116–2125.
von der Lehr N, Johansson S, Wu S, Bahram F, Castell A, Cetinkaya C et al. The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol Cell 2003; 11: 1189–1200.
Choi SH, Wright JB, Gerber SA, Cole MD . Myc protein is stabilized by suppression of a novel E3 ligase complex in cancer cells. Genes Dev 2010; 24: 1236–1241.
Popov N, Schulein C, Jaenicke LA, Eilers M . Ubiquitylation of the amino terminus of Myc by SCF(beta-TrCP) antagonizes SCF(Fbw7)-mediated turnover. Nat Cell Biol 2010; 12: 973–981.
Paul I, Ahmed SF, Bhowmik A, Deb S, Ghosh MK . The ubiquitin ligase CHIP regulates c-Myc stability and transcriptional activity. Oncogene 2013; 32: 1284–1295.
Farrell AS, Sears RC . MYC degradation. Cold Spring Harbor Perspect Med 2014; 4 a014365, 1–15.
Silva JM, Li MZ, Chang K, Ge W, Golding MC, Rickles RJ et al. Second-generation shRNA libraries covering the mouse and human genomes. Nat Genet 2005; 37: 1281–1288.
Li X, Amazit L, Long W, Lonard DM, Monaco JJ, O'Malley BW . Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGgamma-proteasome pathway. Mol Cell 2007; 26: 831–842.
Zhang Z, Clawson A, Realini C, Jensen CC, Knowlton JR, Hill CP et al. Identification of an activation region in the proteasome activator REGalpha. Proc Natl Acad Sci USA 1998; 95: 2807–2811.
Li J, Gao X, Ortega J, Nazif T, Joss L, Bogyo M et al. Lysine 188 substitutions convert the pattern of proteasome activation by REGgamma to that of REGs alpha and beta. EMBO J 2001; 20: 3359–3369.
Conacci-Sorrell M, Ngouenet C, Anderson S, Brabletz T, Eisenman RN . Stress-induced cleavage of Myc promotes cancer cell survival. Genes Dev 2014; 28: 689–707.
Conacci-Sorrell M, Ngouenet C, Eisenman RN . Myc-nick: a cytoplasmic cleavage product of Myc that promotes alpha-tubulin acetylation and cell differentiation. Cell 2010; 142: 480–493.
Liu J, Wang Y, Li L, Zhou L, Wei H, Zhou Q et al. Site-specific acetylation of the proteasome activator REGgamma directs its heptameric structure and functions. J Biol Chem 2013; 288: 16567–16578.
Dang CV . c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol 1999; 19: 1–11.
Chan CH, Lee SW, Li CF, Wang J, Yang WL, Wu CY et al. Deciphering the transcriptional complex critical for RhoA gene expression and cancer metastasis. Nat Cell Biol 2010; 12: 457–467.
Sears R, Ohtani K, Nevins JR . Identification of positively and negatively acting elements regulating expression of the E2F2 gene in response to cell growth signals. Mol Cell Biol 1997; 17: 5227–5235.
Dai MS, Arnold H, Sun XX, Sears R, Lu H . Inhibition of c-Myc activity by ribosomal protein L11. EMBO J 2007; 26: 3332–3345.
Gallant P . Myc function in Drosophila. Cold Spring Harbor Perspect Med 2013; 3: a014324.
Montero L, Muller N, Gallant P . Induction of apoptosis by Drosophila Myc. Genesis 2008; 46: 104–111.
Brand AH, Perrimon N . Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993; 118: 401–415.
Uchimura Y, Barton LF, Rada C, Neuberger MS . REG-gamma associates with and modulates the abundance of nuclear activation-induced deaminase. J Exp Med 2011; 208: 2385–2391.
Kanai K, Aramata S, Katakami S, Yasuda K, Kataoka K . Proteasome activator PA28{gamma} stimulates degradation of GSK3-phosphorylated insulin transcription activator MAFA. J Mol Endocrinol 2011; 47: 119–127.
Okamura T, Taniguchi S, Ohkura T, Yoshida A, Shimizu H, Sakai M et al. Abnormally high expression of proteasome activator-gamma in thyroid neoplasm. J Clin Endocrinol Metab 2003; 88: 1374–1383.
He J, Cui L, Zeng Y, Wang G, Zhou P, Yang Y et al. REGgamma is associated with multiple oncogenic pathways in human cancers. BMC Cancer 2012; 12: 75.
Liu N, Li H, Li S, Shen M, Xiao N, Chen Y et al. The Fbw7/human CDC4 tumor suppressor targets proproliferative factor KLF5 for ubiquitination and degradation through multiple phosphodegron motifs. J Biol Chem 2010; 285: 18858–18867.
Acknowledgements
We are grateful to Dr. Naohiko Ikegaki, Chuangui Wang, Mu-Shui Dai, Dianqing Wu, Lin Li and Hui-Kuan Lin for kindly providing reagents. We thank Bloomington and VDRC stock center for fly strains. We thank other members of the Wang lab for their assistances. This work was supported through grants from the National Basic Research Program of China (973 program 2010CB529704 and 2012CB910404), National Natural Science Foundation of China (30800587, 30971521 and 31171338) and the Science and Technology Commission of Shanghai Municipality (11DZ2260300) to PW and National Natural Science Foundation of China (81071657) and the National Basic Research Program (2009CB918402, 2011CB504200) to XL. PW is a scholar of the Program for New Century Excellent Talents in University (NCET-10-0387) and the Dawn Program of Shanghai Education Commission (11SG27).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by JM Hardwick
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Li, S., Jiang, C., Pan, J. et al. Regulation of c-Myc protein stability by proteasome activator REGγ. Cell Death Differ 22, 1000–1011 (2015). https://doi.org/10.1038/cdd.2014.188
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2014.188
This article is cited by
-
Cold temperature extends longevity and prevents disease-related protein aggregation through PA28γ-induced proteasomes
Nature Aging (2023)
-
Oct4 cooperates with c-Myc to improve mesenchymal-to-endothelial transition and myocardial repair of cardiac-resident mesenchymal stem cells
Stem Cell Research & Therapy (2022)
-
MYC: a multipurpose oncogene with prognostic and therapeutic implications in blood malignancies
Journal of Hematology & Oncology (2021)
-
Reciprocal REGγ-mTORC1 regulation promotes glycolytic metabolism in hepatocellular carcinoma
Oncogene (2021)
-
Expanding the role of proteasome homeostasis in Parkinson’s disease: beyond protein breakdown
Cell Death & Disease (2021)