Abstract
Background:
We conducted the first study to investigate post-diagnostic oral bisphosphonates use and colorectal cancer-specific mortality.
Methods:
Colorectal cancer patients were identified from the National Cancer Data Repository (1998–2007) and linked to the UK Clinical Practice Research Datalink, providing prescription records, and Office of National Statistics mortality data. Time-dependent Cox regression models investigated colorectal cancer-specific mortality in post-diagnostic bisphosphonate users.
Results:
Overall, in 4791 colorectal cancer patients, there was no evidence of an association between bisphosphonate use and colorectal cancer-specific mortality (adjusted hazard ratio=1.11; 95% confidence interval 0.80, 1.54) or with drug frequency or type.
Conclusions:
In this novel population-based cohort study, post-diagnostic bisphosphonate use was not associated with longer rates of colorectal cancer survival.
Similar content being viewed by others
Main
Bisphosphonates, in particular those containing nitrogen, may act on tumour cells directly protecting against visceral metastases. Studies have reported antiproliferative effects and proapoptotic effects, as well as reductions in tumour cell adhesion, invasion, angiogenesis and immune system modulation (Neville-Webbe et al, 2002). Studies in colorectal cancer (CRC) found similar results reporting decreased cell proliferation (Suri et al, 2001; Sassa et al, 2009) and increased apoptosis (Suri et al, 2001; Sewing et al, 2008).
Clinical trials also suggest bisphosphonates may influence cancer survival. Trials of bisphosphonates as adjuvant therapy in breast cancer patients reported improved survival outcomes, however, evidence is inconsistent (Neville-Webbe et al, 2002). In CRC, studies have shown reductions in risk (Yang et al, 2013), but no epidemiological studies have investigated the effect of bisphosphonates on cancer outcomes in patients diagnosed with CRC.
This was the first study to investigate the effect of post-diagnostic oral bisphosphonate usage on CRC-specific mortality in a large prospective UK population-based cohort of CRC patients.
Materials and methods
Study design
The UK Clinical Practice Research Datalink (CPRD) contains demographic information, clinical diagnoses and details of issued prescriptions. This was linked to the National Cancer Data Repository (NCDR), comprising data from all English cancer registries including date, site of primary cancer diagnosis, stage and treatment data and to the Office of National Statistics (ONS) mortality data up to January 2011. Ethical approval for observational research using CPRD has been obtained from a multicentre research ethics committee. CRC cases were identified from CPRD, based on a primary diagnosis of CRC, confirmed by a NCDR CRC diagnosis (ICD codes C18 for colon and C19/C20 for rectum) from 1998 to 2007. Individuals with a history of cancer were excluded (except in situ neoplasms and non-melanoma skin). Patients were excluded if the date of cancer diagnosis predated their date of registration at a CPRD practice or predated CPRD quality records or occurred after the last date of data collection. One patient recorded as having a prescription for an intravenous bisphosphonate was excluded. Deaths were classified as CRC-specific if the underlying cause of death was C18, C19, C20, C21 or C26. Follow-up started 1 year after a cancer diagnosis, as it is unlikely that post-diagnostic medication use could influence deaths in this time period. Other studies have utilised similar methods (Yu et al, 2014). Patients were followed up to death, end of registration with the GP, last date of data collection from the GP or end of follow-up.
Exposure data
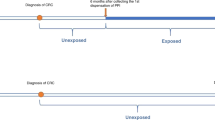
Oral bisphosphonate use was determined from GP prescription records. Prescription data were converted to defined daily doses (DDD) based on quantity and strength in milligrams. Bisphosphonate use was investigated as a time varying covariate (Lévesque et al, 2010), with individuals considered non-users until 6 months after their first prescription. This lag of 6 months removed prescriptions in 6 months before death as these may reflect end of life treatment.
Confounders
NCDR provided data on cancer stage, grade and treatment. Smoking, alcohol consumption and body mass index (BMI) were determined from the closest GP record before a CRC diagnosis (records >10 years were ignored). Pre-diagnostic comorbidities were determined from GP diagnosis codes. Post-diagnostic use of low-dose aspirin, β-blockers, non-steroidal anti-inflammatory drugs (NSAIDs), angiotensin-converting enzyme (ACE) inhibitors and statins (modelled as time varying covariates with a 6 month lag), was determined from GP prescribing data.
Data analysis
Time-dependent Cox regression models were used to calculate hazard ratios (HRs) for cancer-specific deaths and 95% confidence intervals (CIs) for bisphosphonate users vs non-users, adjusting for potential confounders. Analyses were also conducted by DDDs and number of prescriptions, as well as in alendronate users and users of nitrogen-containing bisphosphonates. Analyses were repeated restricting to patients with available stage and grade information and additionally adjusting for these covariates.
Subgroup analyses were conducted by site, stage and among females >60 years. Sensitivity analyses were conducted increasing the lag to 1 year and investigating pre-diagnostic bisphosphonate use in the year before diagnosis, not excluding deaths in the year after diagnosis. Additional analysis investigated post-diagnostic use among new users of bisphosphonates. A propensity score matched analysis was conducted using a simplified analysis based upon use in the first year after diagnosis. Logistic regression was used to create a propensity score for bisphosphonate use in the first year after diagnosis using previously mentioned potential confounders. Matching was implemented by PSMATCH2 (Leuven, 2013) using single nearest neighbour matching. Cox regression compared CRC-specific mortality in bisphosphonate users and propensity score matched control group using a robust variance estimator to account for lack of independence within matched pairs, as recommended (Austin, 2013). All analyses were conducted using STATA 11 (StatCorp, College Station, TX, USA).
On the basis of the observed cohort of 4800 colorectal patients and 1550 cancer-specific deaths and the observed bisphosphonate usage of 7% of individuals, the study would have over 80% power to detect as statistically significant a HR of ∼0.75, using a method based upon Schoenfeld’s log rank test method implemented in STATA 11.
Results
The final cohort consisted of 4791 CRC patients with 1576 CRC-specific deaths. The average follow-up was 3.3 years with a range in follow-up from 1 to 12.9 years. Bisphosphonate users were more likely to be female, to have colon cancer, be older at diagnosis, to be non-drinkers and to have certain comorbidities including chronic obstructive pulmonary disease, congestive heart disease and rheumatological disease (Table 1). In addition, bisphosphonate users were less likely to undergo radiotherapy or chemotherapy compared with non-users and less likely to be current smokers or have a high BMI. Patients using bisphosphonates were also more likely to present with lower-stage disease and were more likely to take other medications (including statins, low-dose aspirin, ACE inhibitors, NSAIDs and β-blockers).
Post-diagnostic bisphosphonate use and colorectal cancer-specific mortality
There was no evidence of an association between CRC-specific death and post-diagnostic bisphosphonate use before (HR=0.94; 95% CI 0.73, 1.22) or after adjustment for potential confounders (fully adjusted HR=1.11 95%; CI 0.80, 1.54) (Table 2). There was no evidence of a dose–response relationship in users of >12 prescriptions (adjusted HR=1.16 95%; CI 0.72, 1.88) or those ⩾365 DDDs (adjusted HR=1.09 95%; CI 0.67, 1.77). The observed association remained similar in users of nitrogen-containing bisphosphonates and was slightly attenuated in those using alendronate (adjusted HR=1.25 95%; CI 0.85, 1.84).
Sensitivity analyses
In most subgroup and sensitivity analyses the observed associations were similar (Table 2). In particular, associations with CRC-specific mortality were similar when propensity score matched analyses were conducted on all confounders (HR=1.13 95%; CI 0.62, 2.07). Use of bisphosphonates in the year before diagnosis gave a more marked estimate (adjusted HR=1.35; 95% CI 0.90, 2.02).
Discussion
This study of a large population-based CRC cohort, found no association between post-diagnostic bisphosphonate use and CRC-specific mortality.
No studies have investigated bisphosphonate use and survival in a cohort of CRC patients. Although previous studies have reported reductions in CRC risk among bisphosphonate users (Yang et al, 2013), these protective associations do not appear to translate to CRC survival. However, a study of post-menopausal women reported that users of alendronate had a reduced risk of dying from colon cancer (adjusted HR=0.62 95%; CI 0.52, 0.72) (Pazianas et al, 2012), but as this cohort was not restricted to colon cancer patients this estimate is likely to largely reflect incidence rather than survival. The authors also reported that alendronate users who developed colon cancer had reduced all-cause mortality compared with alendronate non-users who developed colon cancer (adjusted HR=0.82; 95% CI 0.70, 0.97). However, this estimate will have been influenced by non-cancer mortality and this estimate is based upon alendronate use determined years before colon cancer diagnosis (potentially up to 10 years).
Our study utilised a large cohort of CRC patients and linkage with NCDR and ONS data allowed robust verification of cancer diagnosis and death data, respectively. Using GP prescribing data should capture almost all usage as bisphosphonates are not available over the counter in the UK as well as eliminating any recall bias that exists in questionnaire-based studies and allowing temporal relationships to be investigated. Although consumption cannot be guaranteed, similar findings were observed when assessing increasing number of prescriptions and DDDs, thus reducing the likelihood that compliance is affecting our results. It is possible however, that bias due to misclassification of cancer-specific death could occur. Although we adjusted for important confounders such as sex, stage and treatment, the possibility of residual confounding remains as we were unable to adjust for other confounders such as socioeconomic status. Although bone metastasis is an indication for intravenous bisphosphonates use in the UK (Joint Formulary Committee, 2014), this seems unlikely to bias our results because we investigated only oral bisphosphonates and bone metastases is rare in CRC cancer patients (Roth et al, 2009). Additionally, bisphosphonate users had lower stage at presentation and analysis of bisphosphonate use before diagnosis revealed similar results.
In conclusion, this large population-based study of CRC patients found no association between bisphosphonate use and CRC-specific mortality. Our findings do not support preclinical evidence suggesting bisphosphonates may protect against visceral metastases (Neville-Webbe et al, 2002).
Change history
30 June 2015
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Austin PC (2013) The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med 32: 2837–2849.
Joint Formulary Committee (2014) British National Formulary. BMJ Publishing Group Ltd.
Leuven E, Sianesi B (2013) PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing: version 4.0.5, http://ideas.repec.org/c/boc/bocode/s432001.html.
Lévesque LE, Hanley JA, Kezouh A, Suissa S (2010) Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 340: b5087.
Neville-Webbe H, Holen I, Coleman R (2002) The anti-tumour activity of bisphosphonates. Cancer Treat Rev 28: 305–319.
Pazianas M, Abrahamsen B, Eiken PA, Eastell R, Russell RGG (2012) Reduced colon cancer incidence and mortality in postmenopausal women treated with an oral bisphosphonate—Danish National Register Based Cohort Study. Osteoporos Int 23: 2693–2701.
Roth ES, Fetzer DT, Barron BJ, Joseph Ua, Gayed IW, Wan DQ (2009) Does colon cancer ever metastasize to bone first? a temporal analysis of colorectal cancer progression. BMC Cancer 9: 274.
Sassa S, Okabe H, Nemoto N, Kikuchi H, Kudo H, Sakamoto S (2009) Ibadronate may prevent colorectal carcinogenesis in mice with ulcerative colitis. Anticancer Res 29: 4615–4619.
Sewing L, Steinberg F, Schmidt H, Göke R (2008) The bisphosphonate zoledronic acid inhibits the growth of HCT-116 colon carcinoma cells and induces tumor cell apoptosis. Apoptosis 13: 782–789.
Suri S, Mönkkönen J, Taskinen M, Pesonen J, Blank M, Phipps R, Rogers M (2001) Nitrogen-containing bisphosphonates induce apoptosis of Caco-2 cells in vitro by inhibiting the mevalonate pathway: a model of bisphosphonate-induced gastrointestinal toxicity. Bone 29: 336–343.
Yang G, Hu H, Zeng R, Huang J (2013) Oral bisphosphonates and the risk of colorectal cancer: a meta-analysis. J Clin Gastroenterol 47: 741–748.
Yu O, Eberg M, Benayoun S, Aprikian A, Batist G, Suissa S, Azoulay L (2014) Use of statins and the risk of death in patients with prostate cancer. J Clin Oncol 32: 5–11.
Acknowledgements
BH was funded by Northern Ireland Department of Education and Learning PhD studentship. CRC was supported by a Health and Social Care Research and Development, Public Health Agency, Northern Ireland, funded UK NIHR Career Development Fellowship. Access to the data was funded by a Cancer Research UK project grant (C39066/A14597).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Hicks, B., Murray, L., Hughes, C. et al. Post-diagnostic oral bisphosphonate use and colorectal cancer mortality: a population-based cohort study within the UK Clinical Practice Research Datalink. Br J Cancer 113, 123–126 (2015). https://doi.org/10.1038/bjc.2015.152
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2015.152