Abstract
Schizophrenia is considered primarily as a cognitive disorder. However, functional outcomes in schizophrenia are limited by the lack of effective pharmacological and psychosocial interventions for cognitive impairment. GABA (gamma-aminobutyric acid) interneurons are the main inhibitory neurons in the central nervous system (CNS), and they play a critical role in a variety of pathophysiological processes including modulation of cortical and hippocampal neural circuitry and activity, cognitive function-related neural oscillations (eg, gamma oscillations) and information integration and processing. Dysfunctional GABA interneuron activity can disrupt the excitatory/inhibitory (E/I) balance in the cortex, which could represent a core pathophysiological mechanism underlying cognitive dysfunction in schizophrenia. Recent research suggests that selective modulation of the GABAergic system is a promising intervention for the treatment of schizophrenia-associated cognitive defects. In this review, we summarized evidence from postmortem and animal studies for abnormal GABAergic neurotransmission in schizophrenia, and how altered GABA interneurons could disrupt neuronal oscillations. Next, we systemically reviewed a variety of up-to-date subtype-selective agonists, antagonists, positive and negative allosteric modulators (including dual allosteric modulators) for α5/α3/α2 GABAA and GABAB receptors, and summarized their pro-cognitive effects in animal behavioral tests and clinical trials. Finally, we also discuss various representative histone deacetylases (HDAC) inhibitors that target GABA system through epigenetic modulations, GABA prodrug and presynaptic GABA transporter inhibitors. This review provides important information on current potential GABA-associated therapies and future insights for development of more effective treatments.
Similar content being viewed by others
Introduction
Schizophrenia is a devastating psychiatric disease that affects approximately 1% of the population worldwide. This disorder is characterized by a heterogenous combination of symptoms that can be divided into positive symptoms including delusions and hallucinations, negative symptoms of impaired motivation, social withdrawal and affective flattening, and cognitive deficits such as impairments in attention, reasoning, processing speed as well as verbal and working memory1,2. The onset of psychosis typically occurs in late adolescence or early adulthood, but there are rare cases in which symptoms emerge during childhood or old age. A decline in cognitive ability or prodrome often precedes the onset of the first psychotic episode3. Cognitive symptoms persist throughout the course of the illness, and are the most important factors in long-term functional outcomes in schizophrenia1,2,4,5.
Although schizophrenia is typically classified as a psychotic disorder1,5, some suggest that it should be “considered primarily and foremost as a cognitive disorder”1. Current pharmaceutical treatments are partially effective in reducing positive symptoms, but not for negative and cognitive symptoms. Therefore, functional outcomes in schizophrenia are limited by the lack of effective pharmacological and psychosocial interventions for cognitive impairment1,2,6.
γ-Aminobutyric acid (GABA) interneurons are the main inhibitory neurons in the central nervous system (CNS), and they play a critical role in a variety of physiological processes including modulation of cortical and hippocampal neural circuitry and activity7,8, cognitive function-related neural oscillations (eg gamma oscillations)9 and information integration and processing10. Multiple lines of evidence strongly support that the GABAergic system is a major convergence point for both genetic and environmental risk factors of schizophrenia11. For instance, a recent large genome-wide association study (GWAS) involving 11 355 schizophrenia patients and 16 416 controls identified copy number variations (CNVs) enriched in genes related to GABA neurotransmission12. Duplications were strongly enriched for components of GABAA receptor complexes, where the most highly associated genes were α5, β3, and δ receptor subunits. It is known that excessive α5-GABA receptor signaling can contribute to cognitive impairment of schizophrenia (discussed below), thereby providing strong support for the view that tonic inhibition mediated by α5 and δ subunits-containing GABAA receptors could be disrupted in schizophrenia12. Mutations in schizophrenia-linked genes such as DISC113, NRG1 and ERBB414 can lead to disrupted GABA interneuron development, perturbed GABA circuitry and impaired synchrony of neural oscillations. Environmental factors such as prenatal infection and hypoxia, stress, smoking and cannabis use, when interacting with risk genes, can also contribute to schizophrenia15,16. Dysfunctional GABA interneuron activity can disrupt the excitatory/inhibitory (E/I) balance in the cortex17, which could be a core pathophysiological mechanism underlying cognitive dysfunction in schizophrenia. In this review, we will discuss evidence from postmortem and animal studies for abnormal GABA neurotransmission in schizophrenia, and how altered GABA interneurons could disrupt neuronal oscillations underlying cognitive impairment in schizophrenia. We will also discuss potential therapeutic targets and pharmacological treatments for cognitive deficits, with a focus on selective GABAA and GABAB receptor modulators, epigenetic modulations, GABA prodrug and presynaptic GABA transporter inhibitors.
GABA system and cognitive dysfunction in schizophrenia
Reductions in parvalbumin and GAD67 expression are associated with alterations in GABA transmission in schizophrenia
Several markers of GABA neurotransmission are altered in cortical regions of patients with schizophrenia. The most conserved and consistent finding from multiple imaging, animal and postmortem studies is the reduction in mRNA and protein levels for the 67 kDa isoform of glutamic acid decarboxylase (GAD67). This enzyme is responsible for synthesizing the majority of cytosolic and vesicular GABA18 in the dorsal lateral prefrontal cortex (DLPFC), which is responsible for working memory and selective attention19,20,21. Decreased GAD67 mRNA is selectively observed in a subpopulation of prefrontal cortex (PFC) GABA neurons that express the calcium-binding protein parvalbumin (PV)19,22. Around 45% of PV-mRNA positive neurons have undetectable levels of GAD67 mRNA and such alteration is due to reduction in PV mRNA in neurons rather than a decreased density of PV neurons. By contrast, approximately 10% of PV neurons do not express GAD67 mRNA in healthy controls22,23,24. Furthermore, since the reduction in PV mRNA is also found in non-medicated schizophrenia patients and PV mRNA is not altered in the PFC of primates treated long-term with antipsychotics22,23,24, alterations in PV mRNA are unlikely to be due to antipsychotic drugs.
Parvalbumin functions as a slow calcium buffer, and can influence GABA release. Genetic elimination of this protein leads to various alterations in GABA neurotransmission. Taken together, these findings support the hypothesis that the capacity of PV neurons to synthesize and release GABA is impaired in the cortex of patients with schizophrenia25. The reduction in GAD67 mRNA, however, does not necessarily mean that the GABA concentration is decreased in schizophrenia, because the reduced GAD67 mRNA may also be associated with slower GABA metabolism26. Interestingly, a recent study found that the cerebrospinal fluid concentration of GABA analyzed by high-performance liquid chromatography was significantly reduced in first-episode psychosis patients compared with healthy controls, and patients with low GABA concentrations tend to have poor attention. Therefore, this study provides clinical evidence for a potential role of GABA in an early-stage schizophrenia27.
GABA inhibitory circuit deficits lead to impaired neural oscillations in schizophrenia
Many important brain functions depend on the coordinated activity of large populations of neurons within one region or across brain regions. Neural oscillations in the gamma frequency range (30–80 Hz) in the PFC have been studied extensively because of their strong relationship with complex cognitive functions, and because disruption of gamma oscillations could be an important mechanism underlying cognitive deficits in schizophrenia28. Functionally, gamma-band oscillations in the human PFC increase in proportion to working memory load29. Compared to healthy controls, schizophrenia patients have a marked decrease in the amplitude and phase synchronization of gamma oscillations in the frontal cortex, and they tend to perform poorly on executive and working memory tasks28,30. These deficits in gamma oscillations are observed in schizophrenia patients, independently of antipsychotic medication treatment30,31.
In the hippocampus, gamma-band oscillations originating in the CA3 and CA1 regulate network activity that promotes the encoding of spatial information and formation of episodic memories32. Slow theta-frequency oscillations (4-8 Hz) are complementary to gamma oscillations, and are especially important for episodic memory formation. Both gamma and theta oscillations are observed independently in the cortex and hippocampus but they are also coupled to each other9. Aberrant theta-gamma coupling can affect cognitive function in schizophrenia, such as visuospatial working memory33. In addition, ketamine, a pharmacological agent often to create animal models of schizophrenia, has been shown to alter gamma-theta oscillatory coupling in the hippocampus34. Taken together, these findings support the view that disrupted functional connectivity of cortical and hippocampal neural networks is a core mechanism underlying cognitive impairment in schizophrenia31.
It has been suggested that GABAergic inhibitory circuits play a crucial role for the generation of gamma oscillations and synchrony28. Despite the presence of many cortical interneuron subtypes, only some contribute to the generation of gamma oscillations. Several lines of evidence have linked disturbances in the subset of perisomatic-targeting, fast-spiking, PV-expressing (FS PV) neurons to impaired gamma oscillations and working memory deficits in schizophrenia. The synchronous activity of FS PV neurons can generate gamma oscillations in mice in vivo. Optogenetic stimulation35,36 and inhibition35 of FS PV neurons selectively enhances and suppresses gamma oscillations in vivo, respectively. A subset of the FS PV interneurons, namely the parvalbumin-containing basket neurons (PVBC) that synapse on the somas of target pyramidal neurons and mediate fast, strong and shunting inhibition, are primarily responsible for gamma oscillations37. Networks of these basket neurons connected by gap junctions have been shown to produce large synchronous inhibitory postsynaptic potentials (IPSPs) to pyramidal neurons, thus exerting precise inhibitory control over the temporal coding of information in pyramidal neurons25,37. PV interneurons are also important for intrinsic theta rhythm generation in the hippocampus38. Detailed neuronal mechanisms by which PV neurons mediate neural oscillations have been reviewed elsewhere25,26,28,37,39,40,41,42.
The reduced expression of GAD67 and PV in schizophrenia is accompanied by disinhibition of cortical excitatory neurons and diminished neuronal oscillation and synchrony. For example, GAD6726 and PV43 protein levels are lower in PVBC boutons in DLPFC of human postmortem brain tissues43, and downregulation of these two proteins in PV neurons probably reduces gamma oscillatory activity44 in PFC. In addition, by conditionally knocking out one allele of the Gad1 gene (the gene encoding for GAD67) in PV neurons in rats, Lazarus et al found that the decrease in GAD67 mRNA reduced PV-neuron synaptic output45. This in turn, disinhibited local pyramidal neurons in the DLPFC45. Other subtypes of interneurons such as parvalbumin-expressing chandelier cells (PVChCs) and cholecystokinin-expressing (CCK) interneurons also promote gamma synchronization in vivo46. As a whole, these findings suggest that reduced PV neuron output could result from decreased GABA synthesis and calcium buffering. This could be the cause of impaired gamma oscillations and working memory deficits in schizophrenia. Restoring the activity of GAD67 and PV within PV neurons, in particular PVBC, could be a promising strategy for improving cognitive deficits in schizophrenia43.
Furthermore, interneurons that innervate the perisomatic regions of the targeted pyramidal neurons such as axo-somatic PV basket neurons, axo-axonic PV chandelier neurons and CCK-containing neurons, also appear to be essential for generating and maintaining the fast oscillations (eg, gamma oscillations) and slow theta-frequency oscillations in the hippocampus, which play a critical role in different aspects of episodic memory47.
Alterations in other GABA genes related to GABA neurotransmission
In addition to GAD67 and PV, the expression of other key proteins in GABAergic pathways such as GABA transporter type 1 (GAT-1), REELIN (which is encoded by the gene RELN in humans), NMDA receptor subunits (eg, NR2A, NR3A), nicotine acetylcholine receptor (nAChR) α4 and α7 subunits, and brain-derived neurotrophic factor (BDNF)48 are reduced in the brains of schizophrenia patients49,50.
Reelin is essential for regulating the growth, maturation, synaptic plasticity51 and positioning of interneurons in the developing and adult brain52, and hence plays a crucial role in the pathophysiology of schizophrenia51. The decreased number of dendritic spines observed in postmortem brains of schizophrenia patient is likely due to a deficit in reelin51. At the molecular level, reelin can enhance GABA inhibitory signals through inhibition of GAT-1 internalization and increase KCC2 (potassium chloride cotransporter 2) expression53. Hypermethylation of the RELN promoter can result in silencing of reelin54, with reduced transcription53,54.
As molecular alterations of these proteins have been implicated in the pathophysiology of prefrontal cortex dysfunction, they are potential targets for novel pharmacological interventions.
The GABAA receptor as a therapeutic target in schizophrenia
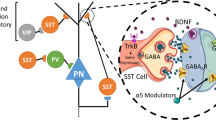
The GABAA receptor is a heteropentameric ligand-gated chloride channel, widely distributed in the mammalian CNS, that mediates synaptic and extrasynaptic inhibition. These receptors are also the site of action of a number of clinically important drugs, including benzodiazepines (BZs), barbiturates, and anesthetics55. GABAARs consist of five different subunits, composed of 19 known subtypes (α1-6, β1-3, γ1-3, δ, ɛ, π, θ and ρ1–3), although three subunits (ρ1–3) are also thought to form the GABAC receptor56. Figure 1 (A) depicts the structure and GABA/benzodiazepine binding sites of GABAA receptors, whereas (B) depicts phasic vs tonic inhibition of the postsynaptic membrane mediated by synaptic vs extrasynaptic GABAA receptors, respectively.
(A) The GABAA receptor generally contains two α, two β and one γ subunit that are arranged in a αβαβγ fashion, of which the γ subunit may be replaced by either an δ, ɛ or θ subunit, and the β subunit may be replaced by σ57. Benzodiazepines enhance the action of the neurotransmitter GABA at GABAA receptors by interaction with their allosteric modulatory benzodiazepine binding site (BZ site) that is formed by one of the α subunits (α1-3 and α5) and γ2 subunit 56. The BZ site is distinct from the endogenous ligand GABA binding site that occurs at the interface of the α and β subunits56,58. The α subunit is of importance in determining the pharmacological properties of the benzodiazepine drugs. (B) GABA-induced chloride ion influx hyperpolarizes postsynaptic neurons, generating inhibitory postsynaptic potentials (IPSPs)56. GABAA receptors mediate two distinct effects on the postsynaptic membrane: synaptic GABAA receptors containing the α1-, α2- and α3- subunits and the ubiquitous γ2 subunit 59 have been shown to possess a low affinity for GABA and mediate fast but short-lasting phasic inhibition, whereas extrasynaptic GABAA receptors with a high-affinity for GABA that contain the relatively rare subunits α4, α5, α6, and δ mediate slow but long-lasting tonic inhibition56,59,60,61,62. The function of phasic inhibition is critically dependent on the synaptic location of the subunit and the IPSP timing. One crucial role of phasic inhibition is to provide timing-based signaling for setting the temporal window of neuron networks firing63, therefore it is important for the generation and regulation of gamma (γ) or theta (θ) oscillations and synchrony64. In contrast, tonic inhibition is responsible for generating about 75% of the total inhibitory conductance received by hippocampal neurons65 and regulates neuronal excitability through its effects on the magnitude and duration of the postsynaptic excitatory postsynaptic potential (EPSP)66.
Benzodiazepine-like ligands for the treatment of cognitive defects in schizophrenia and other neuropsychiatric disorders
In general, the sedative and addictive effects of classical non-selective GABAA receptor benzodiazepines have limited their usefulness in treating psychosis and cognitive impairments in schizophrenia. Consequently, a GABAA receptor subtype-selective compound could overcome these limitations of the classical BZDs without unwanted side effects. Compared to full agonists or antagonists, allosteric modulators like BZDs that possess selective affinity and/or efficacy for different GABAA receptor subtypes have been widely used for various purposes in schizophrenia67. Despite some positive reports, there is no consistent evidence of efficacy of BZDs as adjunctive treatment for positive symptoms in schizophrenia67. Convincing evidence for long-term cognitive benefits is also lacking56.
GABAA receptors containing the α1 subunit are the most prevalent BZDs-sensitive GABAA receptors in the brain68. There is insufficient evidence that selective allosteric agonists at α1 subunit-containing GABAA receptors could improve cognition in schizophrenia68. Two such compounds, triazolam and zolpidem, impair cognition in adult rhesus monkeys in the object retrieval with detours (ORD) task of executive function69. Furthermore, GABAA receptors containing α3 subunits inhibit the dopamine system70, and reduction in α3 subunit expression could contribute to a hyperdopaminergic state in schizophrenia71. Partial positive agonists at α3 subunits (eg, ELB139) could thus be potential antipsychotics70.
Reduction in α5-GABAA receptor signaling might indirectly contribute to increased dopamine signaling in schizophrenia72,73. Therefore, selective allosteric α5 subunit activators could also have potential as antipsychotic medications. Multiple lines of evidence implicate GABAA receptors containing α2 and/or α3 and α5 subunits in cognition. The following sections will focus on these two GABAA subtypes and how selective modulators at these subtypes can improve cognition in neuropsychiatric disorders such as schizophrenia, Alzheimer's disease and Down syndrome.
Role of α5 GABAA receptor in cognitive function
The α5 subtype constitutes 5%–10% of total brain GABAA receptors, but 25% in the hippocampus55. The α5-GABAA receptor is mainly localized in the dendritic regions of the CA1–CA3 hippocampal areas55,74, where they can modulate excitatory glutamatergic input75. α5 subunits are also expressed in olfactory bulb, amygdala, hypothalamus and neocortex (layer V and VI) but to a lesser extent55,74.
Studies in rodents have confirmed the role of α5-GABAA receptors in cognition through a variety of genetic and pharmacological approaches55,59,76. For example, mice deficient for α5 subunits (α5-/-) had better spatial learning in the water maze55. Moreover, α5 (H105R) mutant mice with reduced α5 GABAA receptors on hippocampal pyramidal neurons had stronger trace fear conditioning, but not in hippocampal-independent delay or contextual fear conditioning59.
The α5 GABAA receptor subunit mediates tonic inhibition in hippocampal neurons77, and regulated gamma oscillations77,78. Genetic knock-down or pharmacological inhibition, negative modulation of α5-GABAA receptors promotes hippocampal gamma oscillations77, long-term potentiation (LTP), and learning79. For example, genetic reduction of α5 and δ subunits blocks tonic inhibition in CA3 pyramidal neurons, and produces spontaneous gamma oscillation in vitro. Together, these results suggest that reducing α5-GABA signaling could improve hippocampus-based cognitive functions. However, other studies involving genetic reduction of α5 subunit expression found behavioural abnormalities related to schizophrenia, arguing against the potential therapeutic value of this approach. For example, α5 (H105R) mutant mice also display attenuated prepulse inhibition (PPI), increased spontaneous locomotor activity72 and latent inhibition (LI) deficits.
α5 subtype selective ligands as nootropic drugs
α5 negative allosteric modulators
Inverse agonists at α5-GABAA receptors could have potential to enhance cognition. Several α5 GABAA receptor-selective ligands have tested in animal models. Non-selective BZD inverse agonists may enhance cognitive function in rodents (eg, β3-CCM facilitated spatial learning)80 but can cause anxiety-like behavior (eg, β3-CCM; FG 7142)79,80,81 and seizures (eg, DMCM and FG 7142)58,80,82,83 and cause increased vigilance. These side effects prevent these compounds from being clinically useful79. Reduced expression of α5 GABAA receptors can enhance cognition in some contexts, while α1 subunit inverse agonists can promote seizures. Hence GABAA receptors containing the α5 subunit would appear to be more promising as a target for the development of cognition-enhancing compounds.
The imidazo-benzodiazepine L-655,708 (also known as FG-8094) has weak inverse agonist efficacy at all four GABA receptor subtypes, but a 50–100-fold higher selectivity for the α5 subunit. This drug enhances performance in normal rats in both the learning and recall phases of the water maze, at a dose previously shown to not be pro-convulsant. Unfortunately, the pharmacokinetic profile of this compound makes it unsuitable for further development as a drug. Other similar α5 selective compounds, including RY-023, RY-024 and RY-080, can promote seizures, which obviously precludes them from clinical use79.
To address the side effects associated with targeting GABAA receptors containing the α1 subunit, some have investigated compounds with selectivity at the α5 receptor84. One example of this is RO4938581, an imidazo-triazolo-benzodiazepine with both binding and functional selectivity at the α5 receptor. This compound reversed scopolamine-induced working memory impairment in the delayed match-to-position task (DMTP task) and diazepam-induced spatial learning impairment in the water maze. More importantly, RO4938581 did not produce anxiety or seizures at ∼30% occupancy of hippocampal GABAA α5-receptors84,85. RO4938581 improved performance in a prefrontal cortex-mediated executive function task in monkeys84. This compound also improved cognitive deficits in rats induced by sub-chronic and neonatal administration of phencyclidine (PCP) (a NMDAR antagonist) in novel object recognition (NOR) and intradimensional/extradimensional attentional set-shifting (ID/ED) task, respectively86.
NOR and ID/ED tasks are two preclinical behavioral assays that are related to cognitive tests used to evaluate patients with schizophrenia, such as the MATRICS (measurement and treatment research to improve cognition in schizophrenia) and CNTRICS (cognitive neuroscience treatment research to improve cognition in schizophrenia) test packages61,86,87,88,89,90.
RO4882224, a functional selective inverse agonist for the α5-GABAA receptor from Roche, is another imidazo-triazolo-benzodiazepine. RO4882224 enhanced hippocampal LTP and reversed scopolamine-induced working memory impairment in the DMTP task in rodents85. One derivative of RO4938581, Basmisanil (RG1662 or RO5186582), failed at Phase II clinical trial for the treatment of cognitive impairments in Down syndrome91 and schizophrenia. There is an ongoing Phase II clinical trial with Basmisanil for treating cognitive deficits in schizophrenia (NCT02953639). Other compounds, such as the thiophene MRK-536, enhanced performance in a Morris water maze of spatial memory92 without pro-convulsant effects.
PWZ-029 is another compound with binding selectivity and moderate functional selectivity at α5-containing GABAA receptors. It enhanced encoding and consolidation of memory in normal rats tested with the passive avoidance task at doses that did not cause anxiety-like effects or seizure93, but had no effect in the active avoidance task. Moreover, PWZ-029 attenuated scopolamine-induced impairment of pavlovian fear-conditioned contextual memory in mice94, and reversed scopolamine-induced deficits in novel object recognition, but not the water maze95. PWZ-029 also improved cognitive deficits induced by MK-801 in rodents tested on novel object recognition in the water maze. PWZ-029 did not improve deficits in social recognition memory96. An important caveat with studies of GABAA α5 drugs in animal models is the difficulty in translating the results to schizophrenia, since many features are difficult to accurately reproduce or measure in animals97.
Another compound, α5IA, is a triazolophthalazine with an equivalent affinity for GABAA receptors-containing either an α1, α2, α3 and α5 subunit but with greater inverse agonist efficacy than L-655,708 at the α5 subtype (40% for a5IA vs 17% for L-655,708)79,92,98. α5IA enhances LTP, and improves encoding and recall in the DMTP task, without producing anxiety-like effects or seizure98,99. In addition, α5IA can reverse ethanol-induced memory impairment in healthy volunteers100. However, α5IA actually worsened some cognitive impairments in elderly people98. This drug also has a nephrotoxic metabolite that prevents long-term clinical use in humans. The structurally similar compound α5IA-II has improved oral bioavailability and efficacy selectivity but has pro-convulsant effects92. Another compound MRK-016 (pyrazolotriazine) with high efficacy selectivity at the α5 subtype had promising cognitive effects in animal models but was poorly tolerated in humans92,101.
In summary, inverse agonists at α5 GABAA receptors have cognition-enhancing effects in pre-clinical studies. However, many of these drugs have side effects such as anxiety or seizure, and there is the potential for them to amplify psychotic symptoms since they can impair PPI and latent inhibition in animals.
Dual allosteric modulators of α5 GABAA receptors and α7 nAChRs
The α7 neuronal nicotinic-acetylcholine receptor (α7 nAChR) has been investigated as a valid therapeutic target for treating cognitive deficits in schizophrenia102. Genome-wide association studies have linked deletion of a genetic locus containing the α7 nAChR to increased risk for schizophrenia103. In addition, linkage studies have strongly associated variants in the α7 nAChR gene with deficient P50 auditory gating in schizophrenic patients104. Emerging evidence suggests that P50 sensory gating deficits reflect various cognitive (eg, executive functioning) and perceptual dysfunctions in schizophrenia105.
Interestingly, individuals diagnosed with schizophrenia have among the highest rates of cigarette smoking106. Reduction of α7 nAChR function or expression has been identified as a potential mechanism for elevated tobacco use in schizophrenia107. Moreover, multiple studies have found that nicotine, the major psychoactive component of tobacco, can improve cognitive function (eg, attention and spatial working memory) in schizophrenic patients106,108 by activating α7 nAChRs. Enhancing the activity of α7 nAChRs is a potential strategy for ameliorating cognitive impairment in schizophrenia.
α7 nAChRs are densely expressed in the hippocampus, especially in interneurons where the density of α7 nAChRs is decreased in schizophrenia109. Given the preferential expression of α7 nAChRs in interneurons, the effects of α7 nAChR on hippocampal neurotransmission is mainly mediated by activation of GABA interneurons110. Pre-synaptic α7 receptors facilitate the release of glutamate and GABA102, whereas postsynaptic α7 receptors can regulate GABAA receptor signaling109. α7 nAChR selective positive allosteric modulators (PAMs) are able to preserve the temporal integrity of neurotransmission111 and can improve cognitive deficits in animal models102.
522-054, a novel “dual modulator” that acts both as an α5 GABAA receptor negative allosteric modulator (NAM) and a selective α7 nAChR PAM, was able to restore scopolamine-disrupted deficits in the five-choice serial reaction time test (5-CSRTT) and the eight-arm radial arm maze (RAM). 5-CSRTT is a behavioral test that measures the visual attention and impulsivity in rodents. The test chamber has five holes on one wall, and a reward dispenser on the opposite wall. The task requires the animals to detect a briefly illuminated light presented in one of the five holes and identify the correct spatial location of the hole with nose pokes in order to obtain reward112. The accuracy of visual stimulus discrimination reflects the attentional capacity of the animals112. Intraperitoneal injection of 522-054 in the rats can significantly improve the baseline performance of scopolamine-treated (1.25 mg/kg, ip) animals at a dose of 0.003 mg/kg, suggesting beneficial effects on attention.
The eight-arm RAM task evaluates hippocampus-based spatial learning and memory in rodents. Animals must find the food reward at the end of four randomly chosen maze arms based on spatial navigation cues. At a dose of 0.03 mg/kg, 522-054 had a trend towards reversing the acquired short-term and long-term memory impairments caused by scopolamine (1 mg/kg, ip) in rats.
Flumazenil (an α1- and α5-subunit-selective antagonist) and methyllycaconitine (a selective α7 nAChR antagonist) blocked the effect of 522-054 on scopolamine-induced attentional and cognitive deficits, suggesting that simultaneous allosteric modulation of different receptors mediating related functions can have synergistic effects on cognition111. It is possible that compounds with relatively low specificity and moderate potency could be effective113.
α5 positive allosteric modulators
α5-GABAA receptors mediate the majority of tonic inhibition in hippocampal neurons, and some have suggested that reduced GABA inhibitory input could lead to hyperactivity in the ventral hippocampal dopamine system114. Neural activity in the PFC can synchronize with the neural oscillations in ventral hippocampus, so it is possible that PAMs at α5 GABAA receptors could restore dopamine tone by selectively reducing vHPC output and vHPC-PFC oscillatory activity, with positive effects on cognition115. PAMs selective for GABAA α5 receptors improve hippocampal-dependent memory in a rodent model of age-related memory impairment where CA3 neurons have excess firing rates116. In contrast, other α5-selective PAMs can worsen cognition.
For example, in methylazoxymethanol acetate (MAM)-treated rats, the α5-selective partial agonist SH-053-2′F-R-CH3 impaired cognitive performance58 but normalized the aberrant increase in the number of spontaneously firing dopamine neurons in the VTA to levels comparable to saline-treated rats, and reduced locomotor response to amphetamine117. SH-053-2′F-R-CH3 has no effect on visual recognition and spatial working memory in rhesus monkeys118. Similarly, in an immune-neurodevelopmental model of schizophrenia, the S-isomer of SH-053-2′F-R-CH3 (SH-053-2′F-S-CH3) has detrimental effects on both cognitive function and social interaction119. In contrast, SH-053-2′F-S-CH3 reduces amphetamine-induced hyperactivity119. These findings suggest that α5-selective PAMs are not suitable for treating cognitive deficits in schizophrenia. However, positive modulation at α5 GABAA receptors could be a promising adjunctive treatment for targeting positive symptoms in schizophrenia.
Cognitive enhancement through α2 and/or α3 GABAA receptor modulation
GABAA-α2 receptor and schizophrenia
GABAA receptors containing α2 subunits comprise 15%–20% of all GABAA receptors120. Within the cortex, α2-containing GABAA receptors are enriched on the axon-initial segments (AIS) and perisomatic region of pyramidal neurons that are opposed to PVChC and CCK-basket cell (CCK-BCs) terminals, respectively121. PVChCs have arrays of boutons (cartridges) immunoreactive for the GABA membrane transporter 1 (GAT-1) that innervate the AIS of postsynaptic target neurons122. PVChCs are important for facilitating synchronization of large populations of pyramidal neurons and are thus critical for working memory122. In addition, animal studies have supported an important role for α2-containing GABAA receptors in schizophrenia-related cognitive impairment. For example, mice with down-regulated α2 subunits in the frontal cortex have PPI deficits, reduced gamma oscillations, and impairments in working memory121.
Postmortem and animal studies have reported that the density of PVChC axon cartridges appears to be reduced in DLPFC layer 2-4 in schizophrenia26,122,123. Moreover, the immunoreactivity for the GABAA receptor α2 subunit is markedly elevated in schizophrenia, while the density of α2-labeled AIS is negatively correlated with the density of PVChCs cartridges124. These findings may be interpreted as compensatory responses to the diminished presynaptic GABA input25,120. Based on this interpretation, one would predict that augmenting GABA neurotransmission from chandelier neurons through GABAA receptors containing the α2 subunit could restore gamma-frequency synchronized neuronal activity required for working memory125,126. Therefore, a positive allosteric modulation of α2 subunit-containing GABAA receptors could be a promising therapeutic strategy in schizophrenia.
GABAA α2/ α3 positive allosteric modulators
MK-0777 (also known as TPA-023 or L-830982) is a selective partial positive allosteric modulator at α2 and α3 subtypes. MK-0777 can improve performance on several cognitive tasks in patients with schizophrenia. Moreover, MK-0777 improved frontal gamma band power 127. However, a subsequent larger clinical trial (n=60) failed to replicate these promising findings128. Since MK-0777 is a partial agonist of α2 subunits, a more selective agonist with a greater potency at the GABAA receptor-containing α2 subunit might work better128.
There are questions about the role of PVChCs as neural substrates for the reduced frontal gamma oscillations in patients with schizophrenia26. PVChCs may depolarize cortical pyramidal neurons rather than hyperpolarizing them, and so PVChCs may be a potent source of a slow depolarizing current that mimics the type of slow, NMDA-like depolarization of pyramidal cells26. Moreover, the slow kinetics of α2 GABAA receptors does not appear to meet the requirement for the strong and fast inhibition required for gamma-band neural oscillations26,129,130. In contrast, other studies have indicated that α2 GABAA receptors are strongly coupled to theta oscillations26,131. Consequently, the compensatory effects or lower presynaptic GAT-1 and higher postsynaptic GABAA α2 receptors, in response to decreased GABA neurotransmission may in fact increase EPSCs at the AIS. This could be a way to increase excitation and restore the E/I imbalance in schizophrenia26.
GABAB receptor ligands as cognitive enhancers
GABAB receptors and schizophrenia
GABAB receptors are G-protein-coupled receptors consisting of GABBR1 and GABBR2. Unlike GABAAR, GABABRs are found outside the synapse and have high affinity for GABA132. They are widely distributed in the brain and regulate neuronal network activity133, neurodevelopment134 and synaptic plasticity135. Given their prevalence and widespread distribution in the CNS, it is not surprising that dysfunctions of GABAB receptors have been implicated in numerous CNS disorders such as major depression136, schizophrenia137, bipolar disorder138 and seizures.
Multiple lines of evidence implicate GABAB receptors in the pathophysiology of schizophrenia139. GABAB receptors are markedly reduced in the cerebellum139, hippocampus140, entorhinal cortex, inferior temporal cortex141 of patients with schizophrenia compared with healthy controls. GABAB receptors in the entorhinal cortex and hippocampus are important for memory142. Agonist activation of GABAB inhibits neuronal excitation and gamma oscillations143, while antagonists promote theta and gamma oscillations144. Inhibition of postsynaptic GABAB receptors can enhance LTP by lengthening NMDA receptor-mediated currents145. Inhibition of presynaptic GABAB receptor enhances GABA release, thereby decreasing calcium conductance and subsequent GABA release146.
GABAB receptor antagonists
GABAB receptor antagonists can improve cognition145. Proposed mechanisms include facilitating synaptic plasticity and LTP147,148 and entraining neuronal oscillations144,149. For example, intrahippocampal infusion of the antagonist 2-OH saclofen can markedly reverse scopolamine-induced impairments in LTP and Y-maze performance148. Similarly, the antagonists CGP 55845 and CGP 52432 enhance impaired LTP in the dentate gyrus of Ts65Dn mice, a genetic mouse model of Down Syndrome147. Moreover, CGP 55845 significantly increased gamma and theta oscillations in rat brain slices149. Another antagonist SCH 50911 was also found to increase gamma power150.
So far, a variety of GABAB receptor antagonists have displayed cognition-enhancing effects in animal models of psychiatric disorders. Representative GABAB receptor antagonists and their effects on cognition are summarized in Table 1. CGP 36742 (also known as SGS742) was the first151 and the only antagonist tested in clinical trials for mild cognitive impairment. SGS742 enhances cognition in animal models and in clinical phase II trials (Table 1) (For a comprehensive review, see145,151). However, no current data are available with regards to treating cognitive deficits in schizophrenia using SGS742.
Despite evidence for cognitive enhancing effects of GABAB receptor antagonists, few studies have examined these compounds in animal models of schizophrenia. For example, in a recent apomorphine-susceptible (APO-SUS) young rat model displaying schizophrenia-relevant features146, the level of GAD67 and the density of GAD67-positive cells were reduced. However, basal synaptic input to pyramidal neurons was unaltered. In contrast, the paired-pulse ratio (PPR) at longer inter-stimulus intervals was decreased in APO-SUS rats, indicative of a reduced GABA release. This reduction could be caused by enhanced GABAB receptor signaling. Interestingly, the application of CGP 55845 can completely restore the level of PPR and cause a decrease in GABAB signaling. The authors showed that CGP 55845 is likely to act at presynaptic GABAB receptors rather than postsynaptic receptors. Therefore, these findings are in line with the hypothesis that inhibition of presynaptic GABAB receptors could enhance GABA release. Decreased inhibitory drive from interneurons (eg, PV neurons) onto their postsynaptic targeted pyramidal neurons could be a core pathophysiological feature of schizophrenia173, and that reduced GABA neurotransmission is highly correlated to cognitive defects in schizophrenia146. GABAB receptor antagonists require further investigations, especially in animal models related to schizophrenia in order to determine their potential for treating cognitive impairments in schizophrenia.
GABAB receptor agonists
The prototypic GABAB receptor agonist Baclofen is currently the only marketed drug targeting GABAB receptors. Numerous studies have reported that the Baclofen and other GABAB receptor agonists impair cognition in animal models 174. However, these results are not consistent. Baclofen can ameliorate the recognition memory impairment induced by methamphetamine 175 and the spatial working memory deficits induced by chronic cerebral hypoperfusion in rats 176 (for a more comprehensive review, see174). More studies need to be conducted to confirm the effects of Baclofen on cognition.
GABAB receptor allosteric modulators
The data for the effects of PAM and NAM targeting GABAB receptors on cognition is limited. In the mouse passive avoidance cognition paradigm, a selective GABAB receptor PAM GS39783 showed no deleterious effects on cognition in contrast to Baclofen (1 mg/kg), which significantly impaired cognitive performance177. In 2015, the novel compound 7 from Astellas Pharma, a sulfur-containing bicyclic compound functioning as a GABAB receptor PAM, was used for the prevention or treatment of cognitive disorders, schizophrenia and pain (publication number for patent application: WO2015056771)178.
Epigenetic therapies for cognitive impairment
Alterations in epigenetic regulations in schizophrenia
Epigenetic mechanisms (eg, histone modification, chromatin remodeling, DNA methylation) can synergistically interact with genetics53 to mediate GABA system abnormalities in schizophrenia. Prominent genes include GAD1, RELN, BDNF and GABAB353,179. Several excellent reviews discuss epigenetic mechanisms in the neurobiology of neuropsychiatric disorders including schizophrenia51,53,180,181,182. These articles highlight several key findings about epigenetic alterations in schizophrenia.
Histone modifications in schizophrenia are shifted from open chromatin (H3K4-trimethylation, which positively regulates gene expression)53 to repressive histone methylation (H3K27-methylation that negatively regulates gene expression) at GABAergic gene promoters (eg, GAD1) in PFC of some subjects with schizophrenia51. In addition, the expression of histone deacetylases (HDAC) such as HDAC1 that facilitate downregulation of gene expression is increased in schizophrenic brains and correlated with reduced GAD67 expression51,183. Interestingly, overexpression of neuronal HDAC1 in mouse mPFC (but not in dorsal or ventral hippocampus) resulted in schizophrenia-like phenotypes such as impaired short-term memory, PPI and synaptic plasticity184. In fact, many CNS disorders with cognitive impairment also have reduced histone acetylation185.
DNA (cytosine-5)-methyltransferase (DNMT) (eg, DNMT1 and 3a) expression and DNA hypermethylation are abnormally increased in gene promoters in schizophrenia, which consequently results in MecP2-mediated gene silencing of GABAergic candidate genes such as GAD1, RELN, SOX1051 and BDNF51,53. Therefore, epigenetic modulators acting in GABAergic system could remediate the epigenetic alterations observed in schizophrenia.
HDAC and DNMT inhibitors for the treatment of cognitive defects neuropsychiatric disorders
In animal models, treatment with HDAC inhibitors such as trichostatin A (TSA), valproate (VPA) and MS-275 can increase the expression of reelin and GAD67 by activating demethylation49,186. In addition, co-administration of VPA with antipsychotics (eg, clozapine187, olanzapine and quetiapine) can synergistically potentiate VPA-induced promoter demethylation and chromatin remodeling (see review by49) and enhance antipsychotic effects188. VPA can elevate GABA concentration via synthesis, reuptake, and metabolism. In spite of a few positive reports189,190, VPA is likely to be detrimental to cognition as shown in animal studies191. Class I HDACs play an important role in neuronal and brain development192, and are the best-studied HDACs with respect to cognition (for comprehensive review on this topic, see185). HDAC inhibition/deletion facilitates upregulation of a key set of genes involved in cognitive functions and enhances synaptic plasticity and long-term memory193,194.
Class I HDACs HDAC 2 and 3185,188,195,196,197 and Class II HDACs HDAC 5198 and 6188,195,197 could be potential targets for improving cognition in neuropsychiatric disorders. HDAC 1 appears to have inconsistent effects on cognition184,188,195,198,199,200. Representative HDAC inhibitors that have effects on cognition in preclinical and/or clinical studies for a variety of neuropsychiatric disorders are summarized in Table 2. However, these inhibitors are also reported to cause impairment in memory, learning and cognition in some other studies182 in a brain region- and HDAC isoform-specific manner188. Development of selective inhibitors may reduce undesirable side effects, while still retaining pro-cognitive effects. (For comprehensive reviews on Epigenetics in CNS diseases/cognition, see49,182,185,188,201,202.)
Similar to HDAC inhibitors, DNMT (eg, DNMT1 and 3a) inhibitors such as 5AZA and zebularine are also able to restore the expression of reelin and GAD67. But the majority of these compounds or drugs are clinically used for cancer treatment (eg, 5AZA, zebularine, RG108, decitabine). So far, there are few reported studies of DNMT inhibitors in animal models of schizophrenia182.
In summary, the field of epigenetic drugs for the treatment of cognitive impairment in neuropsychiatric diseases such as schizophrenia is at an early stage and development of these drugs for cognition remains a great challenge. Although HDAC and DNMT inhibitors could be of potential therapeutic value in ameliorating cognitive deficits among high-risk individuals with schizophrenia, obstacles such as the lack of subtype- or brain region-specificity188 or capacity to cross the blood-brain-barrier (BBB)182,240, difficulty with the establishment of dose ranges or treatment duration in clinical trials188, and severe toxicity188 have hindered the translation to the clinic.
GABA prodrug with pro-cognitive and antipsychotic effects
BL-1020 (also known as CYP-1020) is being developed by BioLineRx. It is an ester that combines the dopamine antagonism of perphenazine (a typical antipsychotic D2/5-HT2 antagonist drug) with GABA agonist activity241. This drug has promising pro-cognitive and antipsychotic effects in rodent models of schizophrenia242,243 and phase II clinical trials for schizophrenia (NCT00480571, NCT00567710, NCT00722176)241,244.
Preclinical studies indicate that BL-1020 can cross the BBB244 and is less likely to cause neurological or metabolic side effects than current antipsychotics243,244. In phase II a/b clinical trials, patients with chronic schizophrenia or schizoaffective disorder treated with BL-1020 demonstrated significant improvements in cognition and psychotic symptoms. However, the most recent IIb-III clinical trial (NCT01363349) designed to compare the cognitive effects of treatment with CYP-1020 to risperidone was terminated in 2013 because CYP-1020 did not meet its standard efficacy end points. Therefore, future phase II/III clinical trials are required to determine the clinical efficacy of BL-1020 compared with the established antipsychotics such as risperidone or clozapine. Taken together, BL-1020 has shown promising signs as a novel antipsychotic and pro-cognitive compound with excellent therapeutic effects for psychosis and cognitive impairments, and produces fewer side effects that commonly occur with typical and atypical antipsychotic medications. (For review, see244)
GAT-1 inhibitors
GAT-1, the main plasma membrane GABA transporter in brain132, is localized almost exclusively to axon terminals245, which mediates the uptake of extracellular GABA132. This activity is generally thought to terminate the synaptic effects of GABA132. It has been shown that GAT-1-mediated GABA transport regulates GABAB receptor electrophysiological activity through synaptic GABA132. Blockade of GAT-1 can enhance postsynaptic GABABR-mediated IPSPs246 and presynaptic effects (For a detailed review, see132). Therefore, GAT-1 inhibitors may enhance cognition.
In a lipopolysaccharide (LPS)-treated rat model which mimics the prenatal inflammation thought to contribute to schizophrenia247, the GAT-1 inhibitor Tiagabine (TGB) (also known as Gabitril) prevents impairments in LTP and LTD (long-term depression) in male offspring but had no effect on LTD in control rats248. So far, several studies, including double-blind, placebo-controlled trials, have reported that TGB monotherapy or adjunctive therapy for CNS disease (eg, epilepsy) has no negative effects on cognitive function249,250. In contrast, data for cognitive enhancing effects of this drug is lacking. Currently, a phase III clinical trial evaluating the effects of TGB on brain deficits including neurocognitive functions and clinical symptoms during early-stage schizophrenia is in progress (NCT00179465).
Conclusion
In conclusion, the literature reviewed above suggests that restoring pyramidal neuronal inhibition could normalize aberrant cortical and hippocampal neuronal oscillations in schizophrenia. This could ameliorate cognitive impairments such as episodic memory, working memory and executive function in schizophrenia and other neuropsychological disorders. Pharmacological modulation of synaptic or extrasynaptic GABAergic signaling mediated by GABAA and GABAB receptors could restore disrupted neuronal network synchronization within or between brain regions-associated with learning and memory, which can in turn restore E/I imbalance and cognitive deficits in patients with schizophrenia. Electrophysiological measurements such as electroencephalography (EEG) signals, can provide an index of functional connectivity in the brain251, that could serve as endophenotypes for screening candidate cognitive enhancing drugs for schizophrenia.
Overall, most of the potential cognitive-enhancing pharmacological treatments targeting the GABA neurotransmitter system have shown promise in pre-clinical studies with animals. This is also true for α5 subunit-selective negative allosteric modulators. The paucity of data demonstrating therapeutic effects of these drugs in clinical studies, however, has raised questions about how valid these pre-clinical studies are for predicting clinical therapeutic effects in patients. This problem is generic to many aspects of animal models for psychiatric disease and treatment97, and emphasizes the need for continuing development of more powerful translational animal behavioural assays with better predictive validity. One promising approach is the use of touchscreen-based cognitive tests that can deliver cognitive tests that are nearly identical for both humans and rodents252.
Moreover, dual allosteric modulators acting at two different receptors mediating similar functions could produce synergic effects on cognition. This synergistic strategy could reduce the dosage required for achieving optimal therapeutic efficacy, reduce side effects caused by individual drugs, and potentiate the pro-cognitive effects. Therefore, deliberately targeting multiple receptors could be a promising strategy for improving the pharmacological treatment of cognitive impairment in schizophrenia and other neuropsychiatric disorders.
Finally, epigenetic therapies, in particular selective class I HDAC inhibitors, require further modifications in order to increase brain regional selectivity, capacity to cross the blood-rain-barrier, and to reduce systemic toxicity. The epigenetic machinery is difficult to manipulate with specificity, and this is especially problematic for pharmacological manipulation of higher mental functions such as cognition. However, there are examples that show promise, such as inhibiting histone methyltransferases to treat anxiety and depression253. Given the complexity of human cognition, and the heterogeneity of patients with schizophrenia, it is likely that targeting multiple systems and individualizing pharmacological treatment to each patient, is the way forward.
References
Kahn RS, Keefe RS . Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry 2013; 70: 1107–12.
Kahn RS, Sommer IE, Murray RM, Meyer-Lindenberg A, Weinberger DR, Cannon TD, et al. Schizophrenia. Nat Rev Dis Primers 2015; 1: 15067.
Larson MK, Walker EF, Compton MT . Early signs, diagnosis and therapeutics of the prodromal phase of schizophrenia and related psychotic disorders. Expert Rev Neurother 2010; 10: 1347–59.
Lewis DA, Levitt P . Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci 2002; 25: 409–32.
Bora E . Neurodevelopmental origin of cognitive impairment in schizophrenia. Psychol Med 2015; 45: 1–9.
Volk DW, Lewis DA . Early developmental disturbances of cortical inhibitory neurons: contribution to cognitive deficits in schizophrenia. Schizophr Bull 2014; 40: 952–7.
Benes FM, Berretta S . GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuro-psychopharmacology 2001; 25: 1–27.
Kelsom C, Lu W . Development and specification of GABAergic cortical interneurons. Cell Biosci 2013; 3: 19.
Moran LV, Hong LE . High vs low frequency neural oscillations in schizophrenia. Schizophr Bull 2011; 37: 659–63.
Kann O, Papageorgiou IE, Draguhn A . Highly energized inhibitory interneurons are a central element for information processing in cortical networks. J Cereb Blood Flow Metab 2014; 34: 1270–82.
Schmidt MJ, Mirnics K . Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology 2015; 40: 190–206.
Pocklington AJ, Rees E, Walters JT, Han J, Kavanagh DH, Chambert KD, et al. Novel findings from CNVs implicate inhibitory and excitatory signaling complexes in schizophrenia. Neuron 2015; 86: 1203–14.
Lee FH, Zai CC, Cordes SP, Roder JC, Wong AH . Abnormal interneuron development in disrupted-in-schizophrenia-1 L100P mutant mice. Mol Brain 2013; 6: 20.
Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, et al. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature 2010; 464: 1376–80.
Dean K, Murray RM . Environmental risk factors for psychosis. Dialogues Clin Neurosci 2005; 7: 69–80.
Tsuang MT, Stone WS, Faraone SV . Genes, environment and schizophrenia. Br J Psychiatry Suppl 2001; 40: s18–24.
Uhlhaas PJ . High-frequency oscillations in schizophrenia. Clin EEG Neurosci 2011; 42: 77–82.
Patel AB, de Graaf RA, Martin DL, Battaglioli G, Behar KL . Evidence that GAD65 mediates increased GABA synthesis during intense neuronal activity in vivo. J Neurochem 2006; 97: 385–96.
Blum BP, Mann JJ . The GABAergic system in schizophrenia. Int J Neuropsychopharmacol 2002; 5: 159–79.
Akbarian S, Huang HS . Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev 2006; 52: 293–304.
Lett TA, Voineskos AN, Kennedy JL, Levine B, Daskalakis ZJ . Treating working memory deficits in schizophrenia: a review of the neurobiology. Biol Psychiatry 2014; 75: 361–70.
Hashimoto T, Volk D, Eggan S, Pierri J, Sun Z, Sampson A, et al. Altered gene expression in parvalbumin-containing GABA neurons in the prefrontal cortex of subjects with schizophrenia. Schizophr Res 2003; 60: 71.
Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA . Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry 2000; 57: 237–45.
Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci 2003; 23: 6315–26.
Lewis DA . Inhibitory neurons in human cortical circuits: substrate for cognitive dysfunction in schizophrenia. Curr Opin Neurobiol 2014; 26: 22–6.
Lewis DA, Curley AA, Glausier JR, Volk DW . Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 2012; 35: 57–67.
Orhan F, Fatouros-Bergman H, Goiny M, Malmqvist A, Piehl F, Karolinska Schizophrenia Project C, et al. CSF GABA is reduced in first-episode psychosis and associates to symptom severity. Mol Psychiatry 2017. doi: 10.1038/mp.2017.25.
Uhlhaas PJ, Singer W . Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 2010; 11: 100–13.
Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex 2003; 13: 1369–74.
Chen CM, Stanford AD, Mao X, Abi-Dargham A, Shungu DC, Lisanby SH, et al. GABA level, gamma oscillation, and working memory performance in schizophrenia. Neuroimage Clin 2014; 4: 531–9.
Gallinat J, Winterer G, Herrmann CS, Senkowski D . Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin Neurophysiol 2004; 115: 1863–74.
Montgomery SM, Buzsaki G . Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc Natl Acad Sci U S A 2007; 104: 14495–500.
Lynn PA, Sponheim SR . Disturbed theta and gamma coupling as a potential mechanism for visuospatial working memory dysfunction in people with schizophrenia. Neuropsychiatry Electrophysiol 2016; 2: 7.
Caixeta FV, Cornelio AM, Scheffer-Teixeira R, Ribeiro S, Tort AB . Ketamine alters oscillatory coupling in the hippocampus. Sci Rep 2013; 3: 2348.
Sohal VS, Zhang F, Yizhar O, Deisseroth K . Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 2009; 459: 698–702.
Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 2009; 459: 663–7.
Bartos M, Vida I, Jonas P . Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 2007; 8: 45–56.
Amilhon B, Huh CY, Manseau F, Ducharme G, Nichol H, Adamantidis A, et al. Parvalbumin interneurons of hippocampus tune population activity at theta frequency. Neuron 2015; 86: 1277–89.
Gonzalez-Burgos G, Lewis DA . GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull 2008; 34: 944–61.
Gonzalez-Burgos G, Hashimoto T, Lewis DA . Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep 2010; 12: 335–44.
Shin YW, O'Donnell BF, Youn S, Kwon JS . Gamma oscillation in schizophrenia. Psychiatry Investig 2011; 8: 288–96.
Curley AA, Lewis DA . Cortical basket cell dysfunction in schizophrenia. J Physiol 2012; 590: 715–24.
Glausier JR, Fish KN, Lewis DA . Altered parvalbumin basket cell inputs in the dorsolateral prefrontal cortex of schizophrenia subjects. Mol Psychiatry 2014; 19: 30–6.
Volman V, Behrens MM, Sejnowski TJ . Downregulation of parvalbumin at cortical GABA synapses reduces network gamma oscillatory activity. J Neurosci 2011; 31: 18137–48.
Lazarus MS, Krishnan K, Huang ZJ . GAD67 deficiency in parvalbumin interneurons produces deficits in inhibitory transmission and network disinhibition in mouse prefrontal cortex. Cereb Cortex 2015; 25: 1290–6.
Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T . Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo . J Neurosci 2007; 27: 8184–9.
Nyhus E, Curran T . Functional role of gamma and theta oscillations in episodic memory. Neurosci Biobehav Rev 2010; 34: 1023–35.
Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE . Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry 2003; 8: 592–610.
Guidotti A, Auta J, Chen Y, Davis JM, Dong E, Gavin DP, et al. Epigenetic GABAergic targets in schizophrenia and bipolar disorder. Neuropharmacology 2011; 60: 1007–16.
Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, et al. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology 2012; 62: 1574–83.
Gavin DP, Sharma RP . Histone modifications, DNA methylation, and schizophrenia. Neurosci Biobehav Rev 2010; 34: 882–8.
D'Arcangelo G . Reelin in the Years: controlling neuronal migration and maturation in the mammalian brain. Adv Neurosci 2014; 2014: 1–19.
Shrestha S, Offer SM . Epigenetic regulations of GABAergic neurotransmission: relevance for neurological disorders and epigenetic therapy. Med Epigenet 2016; 4: 1–19.
Costa E, Chen Y, Davis J, Dong E, Noh JS, Tremolizzo L, et al. REELIN and schizophrenia: a disease at the interface of the genome and the epigenome. Mol Interv 2002; 2: 47–57.
Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, et al. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci 2002; 22: 5572–80.
Rudolph U, Knoflach F . Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov 2011; 10: 685–97.
Clayton T, Poe MM, Rallapalli S, Biawat P, Savic MM, Rowlett JK, et al. A review of the updated pharmacophore for the Alpha 5 GABA(A) benzodiazepine receptor model. Int J Med Chem 2015; 2015: 430248.
Savic MM, Huang S, Furtmuller R, Clayton T, Huck S, Obradovic DI, et al. Are GABAA receptors containing alpha5 subunits contributing to the sedative properties of benzodiazepine site agonists? Neuropsychopharmacology 2008; 33: 332–9.
Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, et al. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci U S A 2002; 99: 8980–5.
Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW . Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci 2009; 29: 12757–63.
Vinkers CH, Mirza NR, Olivier B, Kahn RS . The inhibitory GABA system as a therapeutic target for cognitive symptoms in schizophrenia: investigational agents in the pipeline. Expert Opin Investig Drugs 2010; 19: 1217–33.
Brickley SG, Mody I . Extrasynaptic GABAA receptors: their function in the CNS and implications for disease. Neuron 2012; 73: 23–34.
Pouille F, Scanziani M . Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 2001; 293: 1159–63.
Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P . Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature 1995; 378: 75–8.
Mody I, Pearce RA . Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci 2004; 27: 569–75.
Farrant M, Nusser Z . Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 2005; 6: 215–29.
Volz A, Khorsand V, Gillies D, Leucht S . Benzodiazepines for schizophrenia. Cochrane Database Syst Rev 2007: CD006391.
Kehne JH, Maynard GD . GABA and schizophrenia. In: Targets and emerging therapies for schizophrenia. Albert JS, Wood MW, editors. USA: John Wiley & Sons, Inc; 2012.
Makaron L, Moran CA, Namjoshi O, Rallapalli S, Cook JM, Rowlett JK . Cognition-impairing effects of benzodiazepine-type drugs: role of GABAA receptor subtypes in an executive function task in rhesus monkeys. Pharmacol Biochem Behav 2013; 104: 62–8.
Mohler H . Molecular regulation of cognitive functions and developmental plasticity: impact of GABAA receptors. J Neurochem 2007; 102: 1–12.
Yee BK, Keist R, von Boehmer L, Studer R, Benke D, Hagenbuch N, et al. A schizophrenia-related sensorimotor deficit links alpha 3-containing GABAA receptors to a dopamine hyperfunction. Proc Natl Acad Sci U S A 2005; 102: 17154–9.
Hauser J, Rudolph U, Keist R, Möhler H, Feldon J, Yee BK . Hippocampal alpha5 subunit-containing GABAA receptors modulate the expression of prepulse inhibition. Mol Psychiatry 2005; 10: 201–7.
Gerdjikov TV, Rudolph U, Keist R, Mohler H, Feldon J, Yee BK . Hippocampal alpha 5 subunit-containing GABA A receptors are involved in the development of the latent inhibition effect. Neurobiol Learn Mem 2008; 89: 87–94.
Nutt D . GABAA receptors: subtypes, regional distribution, and function. J Clin Sleep Med 2006; 2: S7–11.
Rudolph U, Mohler H . Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol 2004; 44: 475–98.
Bailey DJ, Tetzlaff JE, Cook JM, He X, Helmstetter FJ . Effects of hippocampal injections of a novel ligand selective for the alpha 5 beta 2 gamma 2 subunits of the GABA/benzodiazepine receptor on Pavlovian conditioning. Neurobiol Learn Mem 2002; 78: 1–10.
Glykys J, Mann EO, Mody I . Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci 2008; 28: 1421–6.
Towers SK, Gloveli T, Traub RD, Driver JE, Engel D, Fradley R, et al. Alpha 5 subunit-containing GABA A receptors affect the dynamic range of mouse hippocampal kainate-induced gamma frequency oscillations in vitro . J Physiol 2004; 559: 721–8.
Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, Dawson GR . L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for alpha5-containing GABAA receptors. Neuropharmacology 2006; 51: 1023–9.
Chambers MS, Atack JR, Broughton HB, Collinson N, Cook S, Dawson GR, et al. Identification of a novel, selective GABA(A) alpha5 receptor inverse agonist which enhances cognition. J Med Chem 2003; 46: 2227–40.
Dorow R, Horowski R, Paschelke G, Amin M . Severe anxiety induced by FG 7142, a beta-carboline ligand for benzodiazepine receptors. Lancet 1983; 2: 98–9.
Petersen EN . DMCM: a potent convulsive benzodiazepine receptor ligand. Eur J Pharmacol 1983; 94: 117–24.
Savic MM, Obradovic DI, Ugresic ND, Cook JM, Sarma PV, Bokonjic DR . Bidirectional effects of benzodiazepine binding site ligands on active avoidance acquisition and retention: differential antagonism by flumazenil and beta-CCt. Psychopharmacology (Berl) 2005; 180: 455–65.
Ballard TM, Knoflach F, Prinssen E, Borroni E, Vivian JA, Basile J, et al. RO4938581, a novel cognitive enhancer acting at GABAA alpha5 subunit-containing receptors. Psychopharmacology (Berl) 2009; 202: 207–23.
Knust H, Achermann G, Ballard T, Buettelmann B, Gasser R, Fischer H, et al. The discovery and unique pharmacological profile of RO4938581 and RO4882224 as potent and selective GABAA alpha5 inverse agonists for the treatment of cognitive dysfunction. Bioorg Med Chem Lett 2009; 19: 5940–4.
Redrobe JP, Elster L, Frederiksen K, Bundgaard C, de Jong IE, Smith GP, et al. Negative modulation of GABAA alpha5 receptors by RO4938581 attenuates discrete sub-chronic and early postnatal phencyclidine (PCP)-induced cognitive deficits in rats. Psychopharmacology (Berl) 2012; 221: 451–68.
Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry 2004; 56: 301–7.
Barch DM, Braver TS, Carter CS, Poldrack RA, Robbins TW . CNTRICS final task selection: executive control. Schizophr Bull 2009; 35: 115–35.
Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry 2008; 165: 203–13.
Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA . Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther 2009; 122: 150–202.
Benejam B . Dementia symptoms in Down Syndrome. Int Med Rev Down Syndrome 2009; 13: 18–21.
Atack JR . GABAA receptor subtype-selective modulators. II. alpha5-selective inverse agonists for cognition enhancement. Curr Top Med Chem 2011; 11: 1203–14.
Savic MM, Clayton T, Furtmuller R, Gavrilovic I, Samardzic J, Savic S, et al. PWZ-029, a compound with moderate inverse agonist functional selectivity at GABA(A) receptors containing alpha5 subunits, improves passive, but not active, avoidance learning in rats. Brain Res 2008; 1208: 150–9.
Harris D, Clayton T, Cook J, Sahbaie P, Halliwell RF, Furtmuller R, et al. Selective influence on contextual memory: physiochemical properties associated with selectivity of benzodiazepine ligands at GABAA receptors containing the alpha5 subunit. J Med Chem 2008; 51: 3788–803.
Milic M, Timic T, Joksimovic S, Biawat P, Rallapalli S, Divljakovic J, et al. PWZ-029, an inverse agonist selective for alpha(5) GABAA receptors, improves object recognition, but not water-maze memory in normal and scopolamine-treated rats. Behav Brain Res 2013; 241: 206–13.
Timic Stamenic T, Joksimovic S, Biawat P, Stankovic T, Markovic B, Cook JM, et al. Negative modulation of alpha(5) GABAA receptors in rats may partially prevent memory impairment induced by MK-801, but not amphetamine- or MK-801-elicited hyperlocomotion. J Psychopharmacol 2015; 29: 1013–24.
Wong AH, Josselyn SA . Caution when diagnosing your mouse With schizophrenia: the use and misuse of model animals for understanding psychiatric disorders. Biol Psychiatry 2016; 79: 32–8.
Atack JR . Preclinical and clinical pharmacology of the GABAA receptor alpha5 subtype-selective inverse agonist alpha5IA. Pharmacol Ther 2010; 125: 11–26.
Collinson N, Atack JR, Laughton P, Dawson GR, Stephens DN . An inverse agonist selective for alpha5 subunit-containing GABAA receptors improves encoding and recall but not consolidation in the Morris water maze. Psychopharmacology (Berl) 2006; 188: 619–28.
Nutt DJ, Besson M, Wilson SJ, Dawson GR, Lingford-Hughes AR . Blockade of alcohol's amnestic activity in humans by an alpha5 subtype benzodiazepine receptor inverse agonist. Neuropharmacology 2007; 53: 810–20.
Atack JR, Maubach KA, Wafford KA, O'Connor D, Rodrigues AD, Evans DC, et al. In vitro and in vivo properties of 3-tert-butyl-7-(5-methylisoxazol-3-yl)-2-(1-methyl-1H-1,2,4-triazol-5-ylmethoxy)- pyrazolo[1,5-d]-[1,2,4]triazine (MRK-016), a GABAA receptor alpha5 subtype-selective inverse agonist. J Pharmacol Exp Ther 2009; 331: 470–84.
Beinat C, Banister SD, Herrera M, Law V, Kassiou M . The therapeutic potential of alpha7 nicotinic acetylcholine receptor (alpha7 nAChR) agonists for the treatment of the cognitive deficits associated with schizophrenia. CNS Drugs 2015; 29: 529–42.
Stefansson H, Rujescu D, Cichon S, Pietilainen OP, Ingason A, Steinberg S, et al. Large recurrent microdeletions associated with schizophrenia. Nature 2008; 455: 232–6.
Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, et al. Association of promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry 2002; 59: 1085–96.
Yadon CA, Bugg JM, Kisley MA, Davalos DB . P50 sensory gating is related to performance on select tasks of cognitive inhibition. Cogn Affect Behav Neurosci 2009; 9: 448–58.
Smith RC, Warner-Cohen J, Matute M, Butler E, Kelly E, Vaidhyanathaswamy S, et al. Effects of nicotine nasal spray on cognitive function in schizophrenia. Neuropsychopharmacology 2006; 31: 637–43.
Brunzell DH, McIntosh JM . Alpha7 nicotinic acetylcholine receptors modulate motivation to self-administer nicotine: implications for smoking and schizophrenia. Neuropsychopharmacology 2012; 37: 1134–43.
Levin ED, McClernon FJ, Rezvani AH . Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006; 184: 523–39.
Wanaverbecq N, Semyanov A, Pavlov I, Walker MC, Kullmann DM . Cholinergic axons modulate GABAergic signaling among hippocampal interneurons via postsynaptic alpha 7 nicotinic receptors. J Neurosci 2007; 27: 5683–93.
McKay BE, Placzek AN, Dani JA . Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochem Pharmacol 2007; 74: 1120–33.
Johnstone TB, Gu Z, Yoshimura RF, Villegier AS, Hogenkamp DJ, Whittemore ER, et al. Allosteric modulation of related ligand-gated ion channels synergistically induces long-term potentiation in the hippocampus and enhances cognition. J Pharmacol Exp Ther 2011; 336: 908–15.
Bari A, Dalley JW, Robbins TW . The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc 2008; 3: 759–67.
Buccafusco JJ . Multifunctional receptor-directed drugs for disorders of the central nervous system. Neurotherapeutics 2009; 6: 4–13.
Lodge DJ, Grace AA . Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci 2011; 32: 507–13.
Gill KM, Grace AA . The role of alpha5 GABAA receptor agonists in the treatment of cognitive deficits in schizophrenia. Curr Pharm Des 2014; 20: 5069–76.
Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H . Age-associated alterations of hippocampal place cells are subregion specific. J Neurosci 2005; 25: 6877–86.
Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA . A novel alpha5GABA(A)R-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuro-psychopharmacology 2011; 36: 1903–11.
Soto PL, Ator NA, Rallapalli SK, Biawat P, Clayton T, Cook JM, et al. Allosteric modulation of GABAA receptor subtypes:effects on visual recognition and visuospatial working memory in rhesus monkeys [corrected]. Neuropsychopharmacology 2013; 38: 2315–25.
Richetto J, Labouesse MA, Poe MM, Cook JM, Grace AA, Riva MA, et al. Behavioral effects of the benzodiazepine-positive allosteric modulator SH-053-2'F-S-CH(3) in an immune-mediated neurodevelopmental disruption model. Int J Neuropsychopharmacol 2015; 18:pii: pyu055.
Engin E, Liu J, Rudolph U . alpha2-containing GABA(A) receptors: a target for the development of novel treatment strategies for CNS disorders. Pharmacol Ther 2012; 136: 142–52.
Hines RM, Hines DJ, Houston CM, Mukherjee J, Haydon PG, Tretter V, et al. Disrupting the clustering of GABAA receptor alpha2 subunits in the frontal cortex leads to reduced gamma-power and cognitive deficits. Proc Natl Acad Sci U S A 2013; 110: 16628–33.
Lewis DA . The chandelier neuron in schizophrenia. Dev Neurobiol 2011; 71: 118–27.
Bloomfield C, French SJ, Jones DN, Reavill C, Southam E, Cilia J, et al. Chandelier cartridges in the prefrontal cortex are reduced in isolation reared rats. Synapse 2008; 62: 628–31.
Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA . Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex 2002; 12: 1063–70.
Lewis DA, Volk DW, Hashimoto T . Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology (Berl) 2004; 174: 143–50.
Lewis DA, Hashimoto T, Volk DW . Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 2005; 6: 312–24.
Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, et al. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry 2008; 165: 1585–93.
Buchanan RW, Keefe RS, Lieberman JA, Barch DM, Csernansky JG, Goff DC, et al. A randomized clinical trial of MK-0777 for the treatment of cognitive impairments in people with schizophrenia. Biol Psychiatry 2011; 69: 442–9.
Whittington MA, Cunningham MO, LeBeau FE, Racca C, Traub RD . Multiple origins of the cortical gamma rhythm. Dev Neurobiol 2011; 71: 92–106.
Lewis DA, Fish KN, Arion D, Gonzalez-Burgos G . Perisomatic inhibition and cortical circuit dysfunction in schizophrenia. Curr Opin Neurobiol 2011; 21: 866–72.
Klausberger T, Somogyi P . Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 2008; 321: 53–7.
Gonzalez-Burgos G . GABA transporter GAT1: a crucial determinant of GABAB receptor activation in cortical circuits? Adv Pharmacol 2010; 58: 175–204.
Wang Y, Neubauer FB, Luscher HR, Thurley K . GABAB receptor-dependent modulation of network activity in the rat prefrontal cortex in vitro . Eur J Neurosci 2010; 31: 1582–94.
Gaiarsa JL, Porcher C . Emerging neurotrophic role of GABAB receptors in neuronal circuit development. Front Cell Neurosci 2013; 7: 206.
Lei S, McBain CJ . GABAB receptor modulation of excitatory and inhibitory synaptic transmission onto rat CA3 hippocampal interneurons. J Physiol 2003; 546: 439–53.
Ghose S, Winter MK, McCarson KE, Tamminga CA, Enna SJ . The GABAB receptor as a target for antidepressant drug action. Br J Pharmacol 2011; 162: 1–17.
Kantrowitz J, Citrome L, Javitt D . GABA(B) receptors, schizophrenia and sleep dysfunction: a review of the relationship and its potential clinical and therapeutic implications. CNS Drugs 2009; 23: 681–91.
Fatemi SH, Folsom TD, Thuras PD . GABAA and GABAB receptor dysregulation in superior frontal cortex of subjects with schizophrenia and bipolar disorder. Synapse 2017; 71: doi: 10.1002/syn.21973.
Fatemi SH, Folsom TD, Thuras PD . Deficits in GABA(B) receptor system in schizophrenia and mood disorders: a postmortem study. Schizophr Res 2011; 128: 37–43.
Mizukami K, Sasaki M, Ishikawa M, Iwakiri M, Hidaka S, Shiraishi H, et al. Immunohistochemical localization of gamma-aminobutyric acid(B) receptor in the hippocampus of subjects with schizophrenia. Neurosci Lett 2000; 283: 101–4.
Mizukami K, Ishikawa M, Hidaka S, Iwakiri M, Sasaki M, Iritani S . Immunohistochemical localization of GABAB receptor in the entorhinal cortex and inferior temporal cortex of schizophrenic brain. Prog Neuropsychopharmacol Biol Psychiatry 2002; 26: 393–6.
Schultz H, Sommer T, Peters J . The role of the human entorhinal cortex in a representational account of memory. Front Hum Neurosci 2015; 9: 628.
Deng PY, Xiao Z, Yang C, Rojanathammanee L, Grisanti L, Watt J, et al. GABAB receptor activation inhibits neuronal excitability and spatial learning in the entorhinal cortex by activating TREK-2 K+ channels. Neuron 2009; 63: 230–43.
Heaney CF, Kinney JW . Role of GABAB receptors in learning and memory and neurological disorders. Neurosci Biobehav Rev 2016; 63: 1–28.
Iqbal F, Gillani QA . GABAB receptor antagonists as cognition enhancers, in GABAB receptor. Colombo G, Editor. USA: Humana Press; 2016.
Selten MM, Meyer F, Ba W, Valles A, Maas DA, Negwer M, et al. Increased GABAB receptor signaling in a rat model for schizophrenia. Sci Rep 2016; 6: 34240.
Kleschevnikov AM, Belichenko PV, Faizi M, Jacobs LF, Htun K, Shamloo M, et al. Deficits in cognition and synaptic plasticity in a mouse model of Down syndrome ameliorated by GABAB receptor antagonists. J Neurosci 2012; 32: 9217–27.
Liu QY, Wang CY, Cai ZL, Xu ST, Liu WX, Xiao P, et al. Effects of intrahippocampal GABAB receptor antagonist treatment on the behavioral long-term potentiation and Y-maze learning performance. Neurobiol Learn Mem 2014; 114: 26–31.
Johnson NW, Ozkan M, Burgess AP, Prokic EJ, Wafford KA, O'Neill MJ, et al. Phase-amplitude coupled persistent theta and gamma oscillations in rat primary motor cortex in vitro . Neuropharmacology 2017; 119: 141–56.
Marrosu F, Santoni F, Fa M, Puligheddu M, Barberini L, Genugu F, et al. Beta and gamma range EEG power-spectrum correlation with spiking discharges in DBA/2J mice absence model: role of GABA receptors. Epilepsia 2006; 47: 489–94.
Froestl W, Gallagher M, Jenkins H, Madrid A, Melcher T, Teichman S, et al. SGS742: the first GABAB receptor antagonist in clinical trials. Biochem Pharmacol 2004; 68: 1479–87.
Bianchi M, Panerai AE . Reversal of scopolamine-induced amnesia by the GABAB receptor antagonist CGP 35348 in the mouse. Brain Res Cogn Brain Res 1993; 1: 135–6.
Farr SA, Flood JF, Morley JE . The effect of cholinergic, GABAergic, serotonergic, and glutamatergic receptor modulation on posttrial memory processing in the hippocampus. Neurobiol Learn Mem 2000; 73: 150–67.
Pitsikas N, Rigamonti AE, Cella SG, Muller EE . The GABAB receptor and recognition memory: possible modulation of its behavioral effects by the nitrergic system. Neuroscience 2003; 118: 1121–7.
Kumar K, Kaur H, Deshmukh R . Neuroprotective role of GABAB receptor modulation against streptozotocin-induced behavioral and biochemical abnormalities in rats. Neuroscience 2017; 357: 67–74.
Gillani Q, Ali M, Iqbal F . CGP 35348, GABA B receptor antagonist, has a potential to improve neuromuscular coordination and spatial learning in albino mouse following neonatal brain damage. Biomed Res Int 2014; 2014: 295215.
Gillani Q, Iqbal S, Arfa F, Khakwani S, Akbar A, Ullah A, et al. Effect of GABAB receptor antagonist (CGP35348) on learning and memory in albino mice. ScientificWorldJournal 2014; 2014: 983651.
Wu N, Wang F, Jin Z, Zhang Z, Wang LK, Zhang C, et al. Effects of GABAB receptors in the insula on recognition memory observed with intellicage. Behav Brain Funct 2017; 13: 7.
Mondadori C, Jaekel J, Preiswerk G . CGP 36742: the first orally active GABAB blocker improves the cognitive performance of mice, rats, and rhesus monkeys. Behav Neural Biol 1993; 60: 62–8.
Mondadori C, Mobius HJ, Borkowski J . The GABAB receptor antagonist CGP 36,742 and the nootropic oxiracetam facilitate the formation of long-term memory. Behav Brain Res 1996; 77: 223–5.
Mondadori C, Moebius HJ, Zingg M . CGP 36,742, an orally active GABAB receptor antagonist, facilitates memory in a social recognition test in rats. Behav Brain Res 1996; 77: 227–9.
Carletti R LV, Bowery NG . The GABAB antagonist CGP36742 enhances spatial learning performance and antagonises baclofen induced amnesia in mice. Br J Pharmacol 1993; 109: 74.
Nakagawa Y, Takashima T . The GABAB receptor antagonist CGP36742 attenuates the baclofen- and scopolamine-induced deficit in Morris water maze task in rats. Brain Res 1997; 766: 101–6.
Getova D, Bowery NG, Spassov V . Effects of GABAB receptor antagonists on learning and memory retention in a rat model of absence epilepsy. Eur J Pharmacol 1997; 320: 9–13.
Helm KA, Haberman RP, Dean SL, Hoyt EC, Melcher T, Lund PK, et al. GABAB receptor antagonist SGS742 improves spatial memory and reduces protein binding to the cAMP response element (CRE) in the hippocampus. Neuropharmacology 2005; 48: 956–64.
Sabbagh MN . Drug development for Alzheimer's disease: where are we now and where are we headed? Am J Geriatr Pharmacother 2009; 7: 167–85.
Getova D, Bowery NG . The modulatory effects of high affinity GABA(B) receptor antagonists in an active avoidance learning paradigm in rats. Psychopharmacology (Berl) 1998; 137: 369–73.
Getova DP, Bowery NG . Effects of high-affinity GABAB receptor antagonists on active and passive avoidance responding in rodents with gamma-hydroxybutyrolactone-induced absence syndrome. Psychopharmacology (Berl) 2001; 157: 89–95.
Davies CH, Pozza MF, Collingridge GL . CGP 55845A: a potent antagonist of GABAB receptors in the CA1 region of rat hippocampus. Neuropharmacology 1993; 32: 1071–3.
Lasarge CL, Banuelos C, Mayse JD, Bizon JL . Blockade of GABAB receptors completely reverses age-related learning impairment. Neuroscience 2009; 164: 941–7.
Gillani QA, Akbar A, Ali M, Iqbal F . Gender-specific effects of CGP 55845, GABAB receptor antagonist, on neuromuscular coordination, learning and memory formation in albino mouse following neonatal hypoxia-ischemia insult. Neurol Sci 2015; 36: 961–9.
Hirst WD, Babbs AJ, Green A, Minton JA, Shaw TE, Wise A, et al. Pharmacological characterisation of a cell line expressing GABA B1b and GABA B2 receptor subunits. Biochem Pharmacol 2003; 65: 1103–13.
Rotaru DC, Lewis DA, Gonzalez-Burgos G . The role of glutamatergic inputs onto parvalbumin-positive interneurons: relevance for schizophrenia. Rev Neurosci 2012; 23: 97–109.
Bowery NG . Baclofen: therapeutic use and potential of the prototypic GABAB receptor agonist, in GABAB receptor. Colombo G, Editor. Switzerland: Humana Press; 2016. p337–56.
Arai S, Takuma K, Mizoguchi H, Ibi D, Nagai T, Kamei H, et al. GABAB receptor agonist baclofen improves methamphetamine-induced cognitive deficit in mice. Eur J Pharmacol 2009; 602: 101–4.
Luo P, Chen C, Lu Y, Fu T, Lu Q, Xu X, et al. Baclofen ameliorates spatial working memory impairments induced by chronic cerebral hypoperfusion via up-regulation of HCN2 expression in the PFC in rats. Behav Brain Res 2016; 308: 6–13.
Cryan JF, Kelly PH, Chaperon F, Gentsch C, Mombereau C, Lingenhoehl K, et al. Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N,N'-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. J Pharmacol Exp Ther 2004; 310: 952–63.
Mugnaini C, Corelli F . The allosteric modulation of the GABAB receptor: a medicinal chemistry perspective. In: GABAB receptor. Colombo G, Editor. Switzerland: Humana Press; 2016. p33–52.
Costa E, Dong E, Grayson DR, Guidotti A, Ruzicka W, Veldic M . Reviewing the role of DNA (cytosine-5) methyltransferase overexpression in the cortical GABAergic dysfunction associated with psychosis vulnerability. Epigenetics 2007; 2: 29–36.
Tsankova N, Renthal W, Kumar A, Nestler EJ . Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci 2007; 8: 355–67.
Akbarian S . The molecular pathology of schizophrenia–focus on histone and DNA modifications. Brain Res Bull 2010; 83: 103–7.
Szyf M . Prospects for the development of epigenetic drugs for CNS conditions. Nat Rev Drug Discov 2015; 14: 461–74.
Huang HS, Matevossian A, Whittle C, Kim SY, Schumacher A, Baker SP, et al. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J Neurosci 2007; 27: 11254–62.
Bahari-Javan S, Varbanov H, Halder R, Benito E, Kaurani L, Burkhardt S, et al. HDAC1 links early life stress to schizophrenia-like phenotypes. Proc Natl Acad Sci U S A 2017; 114: E4686–E4694.
Graff J, Tsai LH . The potential of HDAC inhibitors as cognitive enhancers. Annu Rev Pharmacol Toxicol 2013; 53: 311–30.
Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J 2001; 20: 6969–78.
Su P, Jiang AL, Liu F . Clozapine modulates the glycogen synthase kinase-3 signaling partly via GABAB receptors. Ann Biol Sci 2017; 5: 14–20.
Hasan A, Mitchell A, Schneider A, Halene T, Akbarian S . Epigenetic dysregulation in schizophrenia: molecular and clinical aspects of histone deacetylase inhibitors. Eur Arch Psychiatry Clin Neurosci 2013; 263: 273–84.
Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, et al. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer's disease. Neuropsychopharmacology 2010; 35: 870–80.
Takuma K, Hara Y, Kataoka S, Kawanai T, Maeda Y, Watanabe R, et al. Chronic treatment with valproic acid or sodium butyrate attenuates novel object recognition deficits and hippocampal dendritic spine loss in a mouse model of autism. Pharmacol Biochem Behav 2014; 126: 43–9.
Hirsch E, Schmitz B, Carreno M . Epilepsy, antiepileptic drugs (AEDs) and cognition. Acta Neurol Scand Suppl 2003; 180: 23–32.
Montgomery RL, Hsieh J, Barbosa AC, Richardson JA, Olson EN . Histone deacetylases 1 and 2 control the progression of neural precursors to neurons during brain development. Proc Natl Acad Sci U S A 2009; 106: 7876–81.
Peixoto L, Abel T . The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology 2013; 38: 62–76.
Bieszczad KM, Bechay K, Rusche JR, Jacques V, Kudugunti S, Miao W, et al. Histone deacetylase inhibition via RGFP966 releases the Brakes on sensory cortical plasticity and the specificity of memory formation. J Neurosci 2015; 35: 13124–32.
Fischer A, Sananbenesi F, Mungenast A, Tsai LH . Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sci 2010; 31: 605–17.
Rumbaugh G, Sillivan SE, Ozkan ED, Rojas CS, Hubbs CR, Aceti M, et al. Pharmacological selectivity within class I histone deacetylases predicts effects on synaptic function and memory rescue. Neuropsychopharmacology 2015; 40: 2307–16.
Wagner FF, Zhang YL, Fass DM, Joseph N, Gale JP, Weiwer M, et al. kinetically selective inhibitors of histone deacetylase 2 (HDAC2) as cognition enhancers. Chem Sci 2015; 6: 804–815.
Bahari-Javan S, Sananbenesi F, Fischer A . Histone-acetylation: a link between Alzheimer's disease and post-traumatic stress disorder? Front Neurosci 2014; 8: 160.
Kim D, Frank CL, Dobbin MM, Tsunemoto RK, Tu W, Peng PL, et al. Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron 2008; 60: 803–17.
Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 2009; 459: 55–60.
Whittle N, Singewald N . HDAC inhibitors as cognitive enhancers in fear, anxiety and trauma therapy: where do we stand? Biochem Soc Trans 2014; 42: 569–81.
Bredy TW, Barad M . The histone deacetylase inhibitor valproic acid enhances acquisition, extinction, and reconsolidation of conditioned fear. Learn Mem 2008; 15: 39–45.
Camp MC, Macpherson KP, Lederle L, Graybeal C, Gaburro S, Debrouse LM, et al. Genetic strain differences in learned fear inhibition associated with variation in neuroendocrine, autonomic, and amygdala dendritic phenotypes. Neuropsychopharmacology 2012; 37: 1534–47.
Whittle N, Schmuckermair C, Gunduz Cinar O, Hauschild M, Ferraguti F, Holmes A, et al. Deep brain stimulation, histone deacetylase inhibitors and glutamatergic drugs rescue resistance to fear extinction in a genetic mouse model. Neuropharmacology 2013; 64: 414–23.
Wei J, Xiong Z, Lee JB, Cheng J, Duffney LJ, Matas E, et al. Histone modification of Nedd4 ubiquitin ligase controls the loss of AMPA receptors and cognitive impairment induced by repeated stress. J Neurosci 2016; 36: 2119–30.
Fass DM, Reis SA, Ghosh B, Hennig KM, Joseph NF, Zhao WN, et al. Crebinostat: a novel cognitive enhancer that inhibits histone deacetylase activity and modulates chromatin-mediated neuroplasticity. Neuropharmacology 2013; 64: 81–96.
Zhang L, Liu C, Wu J, Tao JJ, Sui XL, Yao ZG, et al. Tubastatin A/ACY-1215 improves cognition in Alzheimer's disease transgenic mice. J Alzheimers Dis 2014; 41: 1193–205.
Graff J, Joseph NF, Horn ME, Samiei A, Meng J, Seo J, et al. Epigenetic priming of memory updating during reconsolidation to attenuate remote fear memories. Cell 2014; 156: 261–76.
Hanson JE, La H, Plise E, Chen YH, Ding X, Hanania T, et al. SAHA enhances synaptic function and plasticity in vitro but has limited brain availability in vivo and does not impact cognition. PLoS One 2013; 8: e69964.
Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, et al. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron 2004; 42: 947–59.
Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci 2007; 27: 6128–40.
Sharma S, Taliyan R, Ramagiri S . Histone deacetylase inhibitor, trichostatin A, improves learning and memory in high-fat diet-induced cognitive deficits in mice. J Mol Neurosci 2015; 56: 1–11.
Sung YM, Lee T, Yoon H, DiBattista AM, Song JM, Sohn Y, et al. Mercaptoacetamide-based class II HDAC inhibitor lowers Abeta levels and improves learning and memory in a mouse model of Alzheimer's disease. Exp Neurol 2013; 239: 192–201.
Govindarajan N, Agis-Balboa RC, Walter J, Sananbenesi F, Fischer A . Sodium butyrate improves memory function in an Alzheimer's disease mouse model when administered at an advanced stage of disease progression. J Alzheimers Dis 2011; 26: 187–97.
Reolon GK, Maurmann N, Werenicz A, Garcia VA, Schroder N, Wood MA, et al. Posttraining systemic administration of the histone deacetylase inhibitor sodium butyrate ameliorates aging-related memory decline in rats. Behav Brain Res 2011; 221: 329–32.
Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH . Recovery of learning and memory is associated with chromatin remodelling. Nature 2007; 447: 178–82.
Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science 2010; 328: 753–6.
Rane P, Shields J, Heffernan M, Guo Y, Akbarian S, King JA . The histone deacetylase inhibitor, sodium butyrate, alleviates cognitive deficits in pre-motor stage PD. Neuropharmacology 2012; 62: 2409–12.
Valvassori SS, Varela RB, Arent CO, Dal-Pont GC, Bobsin TS, Budni J, et al. Sodium butyrate functions as an antidepressant and improves cognition with enhanced neurotrophic expression in models of maternal deprivation and chronic mild stress. Curr Neurovasc Res 2014; 11: 359–66.
Sandner G, Host L, Angst MJ, Guiberteau T, Guignard B, Zwiller J . The HDAC inhibitor phenylbutyrate reverses effects of neonatal ventral hippocampal lesion in rats. Front Psychiatry 2011; 1: 153.
Ricobaraza A, Cuadrado-Tejedor M, Perez-Mediavilla A, Frechilla D, Del Rio J, Garcia-Osta A . Phenylbutyrate ameliorates cognitive deficit and reduces tau pathology in an Alzheimer's disease mouse model. Neuropsychopharmacology 2009; 34: 1721–32.
Wiley JC, Pettan-Brewer C, Ladiges WC . Phenylbutyric acid reduces amyloid plaques and rescues cognitive behavior in AD transgenic mice. Aging Cell 2011; 10: 418–28.
Gardian G, Browne SE, Choi DK, Klivenyi P, Gregorio J, Kubilus JK, et al. Neuroprotective effects of phenylbutyrate in the N171-82Q transgenic mouse model of Huntington's disease. J Biol Chem 2005; 280: 556–63.
Zhou W, Bercury K, Cummiskey J, Luong N, Lebin J, Freed CR . Phenylbutyrate up-regulates the DJ-1 protein and protects neurons in cell culture and in animal models of Parkinson disease. J Biol Chem 2011; 286: 14941–51.
Ristic AJ, Vojvodic N, Jankovic S, Sindelic A, Sokic D . The frequency of reversible parkinsonism and cognitive decline associated with valproate treatment: a study of 364 patients with different types of epilepsy. Epilepsia 2006; 47: 2183–5.
Nicolai J, Aldenkamp AP, Huizenga JR, Teune LK, Brouwer OF . Cognitive side effects of valproic acid-induced hyperammonemia in children with epilepsy. J Clin Psychopharmacol 2007; 27: 221–4.
Shah RD, Jagtap JC, Mruthyunjaya S, Shelke GV, Pujari R, Das G, et al. Sodium valproate potentiates staurosporine-induced apoptosis in neuroblastoma cells via Akt/survivin independently of HDAC inhibition. J Cell Biochem 2013; 114: 854–63.
Wu P, Hong S, Zhong M, Guo Y, Chen H, Jiang L . Effect of sodium valproate on cognitive function and hippocampus of rats after convulsive status epilepticus. Med Sci Monit 2016; 22: 5197–205.
Bowers ME, Xia B, Carreiro S, Ressler KJ . The class I HDAC inhibitor RGFP963 enhances consolidation of cued fear extinction. Learn Mem 2015; 22: 225–31.
Suelves N, Kirkham-McCarthy L, Lahue RS, Gines S . A selective inhibitor of histone deacetylase 3 prevents cognitive deficits and suppresses striatal CAG repeat expansions in Huntington's disease mice. Sci Rep 2017; 7: 6082.
McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, et al. HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci 2011; 31: 764–74.
Jia H, Kast RJ, Steffan JS, Thomas EA . Selective histone deacetylase (HDAC) inhibition imparts beneficial effects in Huntington's disease mice: implications for the ubiquitin-proteasomal and autophagy systems. Hum Mol Genet 2012; 21: 5280–93.
Castellano JF, Fletcher BR, Patzke H, Long JM, Sewal A, Kim DH, et al. Reassessing the effects of histone deacetylase inhibitors on hippocampal memory and cognitive aging. Hippocampus 2014; 24: 1006–16.
Green KN, Steffan JS, Martinez-Coria H, Sun X, Schreiber SS, Thompson LM, et al. Nicotinamide restores cognition in Alzheimer's disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J Neurosci 2008; 28: 11500–10.
Naviaux JC, Schuchbauer MA, Li K, Wang L, Risbrough VB, Powell SB, et al. Reversal of autism-like behaviors and metabolism in adult mice with single-dose antipurinergic therapy. Transl Psychiatry 2014; 4: e400.
Naviaux JC, Wang L, Li K, Bright AT, Alaynick WA, Williams KR, et al. Antipurinergic therapy corrects the autism-like features in the Fragile X (Fmr1 knockout) mouse model. Mol Autism 2015; 6: 1.
Naviaux RK, Curtis B, Li K, Naviaux JC, Bright AT, Reiner GE, et al. Low-dose suramin in autism spectrum disorder: a small, phase I/II, randomized clinical trial. Ann Clin Transl Neurol 2017; 4: 491–505.
Cuadrado-Tejedor M, Garcia-Barroso C, Sanchez-Arias JA, Rabal O, Perez-Gonzalez M, Mederos S, et al. A first-in-class small-molecule that acts as a dual inhibitor of HDAC and PDE5 and that rescues hippocampal synaptic impairment in Alzheimer's disease mice. Neuropsychopharmacology 2017; 42: 524–39.
Ganai SA, Ramadoss M, Mahadevan V . Histone deacetylase (HDAC) inhibitors–emerging roles in neuronal memory, learning, synaptic plasticity and neural regeneration. Curr Neuropharmacol 2016; 14: 55–71.
Kazantsev AG, Thompson LM . Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov 2008; 7: 854–68.
Geffen Y, Keefe R, Rabinowitz J, Anand R, Davidson M . Bl-1020, a new gamma-aminobutyric acid-enhanced antipsychotic: results of 6-week, randomized, double-blind, controlled, efficacy and safety study. J Clin Psychiatry 2012; 73: e1168–74.
Nudelman A, Gil-Ad I, Shpaisman N, Terasenko I, Ron H, Savitsky K, et al. A mutual prodrug ester of GABA and perphenazine exhibits antischizophrenic efficacy with diminished extrapyramidal effects. J Med Chem 2008; 51: 2858–62.
Geffen Y, Nudelman A, Gil-Ad I, Rephaeli A, Huang M, Savitsky K, et al. BL-1020: a novel antipsychotic drug with GABAergic activity and low catalepsy, is efficacious in a rat model of schizophrenia. Eur Neuropsychopharmacol 2009; 19: 1–13.
Fitzgerald PB . BL-1020, an oral antipsychotic agent that reduces dopamine activity and enhances GABAA activity, for the treatment of schizophrenia. Curr Opin Investig Drugs 2010; 11: 92–100.
Conti F, Melone M, Fattorini G, Bragina L, Ciappelloni S . A role for GAT-1 in presynaptic GABA homeostasis? Front Cell Neurosci 2011; 5: 2.
Jensen K, Chiu CS, Sokolova I, Lester HA, Mody I . GABA transporter-1 (GAT1)-deficient mice: differential tonic activation of GABAA versus GABAB receptors in the hippocampus. J Neurophysiol 2003; 90: 2690–701.
Wilson C . Terry AV Jr. Neurodevelopmental animal models of schizophrenia: role in novel drug discovery and development. Clin Schizophr Relat Psychoses 2010; 4: 124–37.
Rideau Batista Novais A, Crouzin N, Cavalier M, Boubal M, Guiramand J, Cohen-Solal C, et al. Tiagabine improves hippocampal long-term depression in rat pups subjected to prenatal inflammation. PLoS One 2014; 9: e106302.
Aikia M, Jutila L, Salmenpera T, Mervaala E, Kalviainen R . Comparison of the cognitive effects of tiagabine and carbamazepine as monotherapy in newly diagnosed adult patients with partial epilepsy: pooled analysis of two long-term, randomized, follow-up studies. Epilepsia 2006; 47: 1121–7.
Salat K, Podkowa A, Mogilski S, Zareba P, Kulig K, Salat R, et al. The effect of GABA transporter 1 (GAT1) inhibitor, tiagabine, on scopolamine-induced memory impairments in mice. Pharmacol Rep 2015; 67: 1155–62.
Lopes da Silva F . EEG and MEG: relevance to neuroscience. Neuron 2013; 80: 1112–28.
Nithianantharajah J, Komiyama NH, McKechanie A, Johnstone M, Blackwood DH, St Clair D, et al. Synaptic scaffold evolution generated components of vertebrate cognitive complexity. Nat Neurosci 2013; 16: 16–24.
Wang DY, Kosowan J, Samsom J, Leung L, Zhang KL, Li YX, et al. Inhibition of the G9a/GLP histone methyltransferase modulates anxiety-related behavior in the mouse. Acta Pharmacol Sin 2018; 39. In press. doi: 10.1038/aps.2017.190.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Xu, My., Wong, A. GABAergic inhibitory neurons as therapeutic targets for cognitive impairment in schizophrenia. Acta Pharmacol Sin 39, 733–753 (2018). https://doi.org/10.1038/aps.2017.172
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2017.172
Keywords
This article is cited by
-
The Roles of hnRNP Family in the Brain and Brain-Related Disorders
Molecular Neurobiology (2023)
-
Structural and stereochemical determinants for hGAT3 inhibition: development of novel conformationally constrained and substituted analogs of (S)-isoserine
Medicinal Chemistry Research (2023)
-
The emerging role of furin in neurodegenerative and neuropsychiatric diseases
Translational Neurodegeneration (2022)
-
Evolutionarily conservative and non-conservative regulatory networks during primate interneuron development revealed by single-cell RNA and ATAC sequencing
Cell Research (2022)
-
Behavioral and Cognitive Consequences of Obesity in Parents and Offspring in Female and Male Rats: Implications of Neuroinflammation and Neuromodulation
Molecular Neurobiology (2022)