Abstract
Aim:
Krüppel-like factor 8 (KLF8) plays important roles in cell cycle and oncogenic transformation. On other hand, androgen receptor (AR) is crucial in development of both androgen-dependent and independent prostatic malignancies. The aim of this study is to investigate the role of KLF8 in prostate cancer (PCa) and the relationship between KLF8 and AR.
Methods:
Eight human PCa cell lines, including androgen-dependent LNCap cells and androgen-independent 22Rv1 cells, as well as human PCa samples were studied. LNCap cells and 22Rv1 cells were transfected with plasmids encoding full-length wild-type KLF8 or KLF8 shRNA. The expression of KLF8 protein was detected using Western blotting or immunohistochemical staining. Cell proliferation in vitro was measured with MTT assay, and in vivo in a xenograft nude mouse model. Yeast two-hybrid screening, co-immunoprecipitation and pull down assays were used to examine the binding of KLF8 to AR. Luciferase reporter gene assay was used to measure the transcriptional activity of the genes targeted by AR.
Results:
In 133 human PCa samples, KLF8 protein staining was observed in 92.65% (63/68) of high-grade PCa, 66.15% (43/65) of low-grade PCa, and 6.82% (3/44) of adjacent normal tissues. The expression of KLF8 was significantly associated with poorer overall survival. Overexpression of KLF8 enhanced the proliferation of both LNCap and 22Rv1 cells, while knockdown of endogenous KLF8 suppressed the proliferation. These manipulations exerted similar effects on the tumor volumes in the xenograft nude mouse model. Yeast two-hybrid screening revealed that KLF8 was a novel AR-interacting protein. With pull down assay and co-immunoprecipitation assay, we demonstrated that KLF8 bound directly to AR, and KLF8 enhanced AR target gene transcription.
Conclusion:
The results demonstrate that KLF8 is a novel AR transcriptional co-activator that is overexpressed in PCa and may play a role in progression of hormone-refractory PCa.
Similar content being viewed by others
Introduction
Prostate cancer (PCa) is a frequently diagnosed cancer and a leading cause of cancer death in men1. AR is a member of the nuclear hormone receptor family of transcriptional factors that activates the expression of numerous androgen-responsive genes, and AR plays key role in the initiation and development of PCa2,3,4,5. Upon androgen binding, AR is released from heat shock proteins (HSPs), homodimerizes, and translocates to the nucleus, where it recruits transcriptional machinery components, chromatin-remodeling complexes, and specific transcriptional co-activators to regulate down-stream transcriptional activities6,7,8,9. For early stage PCa, androgen ablation is a successful therapy to achieve tumor regression. However, in later-stage PCa, AR is usually continuously activated in the absence of androgens, becoming androgen independent10. Currently, there are no curative therapeutic agents for this hormone-resistant stage of PCa11.
The Krüppel-like factor (KLF) family of transcription factors share homology in three C2-H2 zinc finger DNA binding domains. KLF8, a member of the KLF family, is highly expressed and plays important roles in many human malignant tumors; it also plays a critical role in the regulation of cell cycle progression, oncogenic transformation and tumor cell dissemination12,13,14,15,16. KLF8 was recently shown to induce the epithelial-to-mesenchymal transition (EMT) in tumor cells and plays a crucial role in human carcinoma metastasis17. However, the role of KLF8 in PCa is unknown.
Here, we report that KLF8 expression was significantly associated with poorer overall survival in PCa patients. Overexpression of KLF8 enhanced PCa cell growth, whereas knockdown of KLF8 inhibited PCa cell growth, both in vitro and in vivo. In this study, we identify KLF8 as a novel AR co-activator and suggest a role for KLF8 in cancer progression.
Materials and methods
Immunohistochemical staining
The study was approved by the Ethics Committee of Harbin Medical University. Human PCa samples were obtained from the Fourth Affiliated Hospital of Harbin Medical University. The pathological grade of tumors was defined according to the Gleason Grading System. Tissue sections with a thickness of 5 μm were dewaxed, and endogenous peroxidase was quenched with 3% H2O2 in methanol for 30 min. After blocking by incubation with 10% BSA in PBS at 37 °C for 1 h, the tissue sections were incubated with anti-KLF8 antibodies in PBS containing 10% BSA at 4 °C overnight, followed by incubation with a horseradish peroxidase-conjugated anti-rabbit antibody. Color was then developed by incubation with an ImmunoPure Metal Enhanced Diaminobenzidine (DAB) Substrate kit (Pierce). The tissue sections were washed three times in PBS for 10 min after each incubation. The tissue sections were finally counterstained with hematoxylin. To determine KLF8 immunoreactivity, cytosolic or nuclear staining of yellowish or brownish granules was graded as follows: 0 for background staining, 1 for faint staining, 2 for moderate staining, and 3 for strong staining. In addition, positively stained areas in entire tissue sections were graded as follows: 0 for <5%, 1 for 5%–25%, 2 for 26%–50%, 3 for 51%–75%, and 4 for 75%–100%. When combining these two parameters, 0–2 and ≥3 were considered negative and positive staining, respectively. The PSA value was determined with Plus-180 (Bayer, Pittsburgh, PA, USA).
Plasmids and antibodies
KLF8 cDNA and KLF8 short hairpin RNA (shRNA) were purchased from Open Biosystems. The KLF8 shRNA target sequence was 5′-CTGGTCGATATGGATAAACTCA-3′, and the nonsense shRNA sequence was 5′-AGTGCACGTGCATGTCCTA-3′. The rabbit anti-KLF8 antibody that was used for the Western blot assay, immunoprecipitation assay and immunohistochemical assay was purchased from Abcam. The rabbit anti-AR antibody used in the Western blot assay was purchased from Abcam. The mouse anti-α-tubulin antibody used in the Western blotting assay was purchased from Sigma. MTT assay reagents were purchased from DingGuo Biotech.
Cell culture
The following human PCa cell lines were purchased from American Type Culture Collection (ATCC): LNCap, 22Rv1, LAPC-4, LAPC-9, PC3, DU145, RWPE-1, and Vcap. The cell lines were cultured according to the recommendations of the ATCC. LNCap and 22Rv1 cells were transfected with plasmids encoding full-length, wild-type of KLF8 or a control vector, KLF8 shRNA or Ctrl shRNA using Lipofectamine 2000 (Invitrogen). Cells were selected with 0.8 mg/mL G418 (GIBCO BRL) for two weeks, and cell pools in which KLF8 was stably overexpressed or knocked down were obtained for the corresponding assays.
Yeast 2-hybrid assay
AR was identified from a prostate library (Clontech) in a yeast two-hybrid screen using the KLF8 bait clone pGBKT7-KLF8 (containing full-length KLF8).
Immunoblotting, immunoprecipitation and GST pull down
For the immunoblotting assay, cells lysates were subjected to SDS-PAGE, transferred to PVDF membranes (Millipore) and detected with appropriate primary antibodies. Anti-mouse- or anti-rabbit-HRP-conjugated secondary antibodies (Sigma) were used, and the blotting signals were detected with SuperSignal West Dura Extended Duration Substrate (Pierce). Immunoblotting signals were quantitatively analyzed via densitometry analysis with LAS4000 Image software (Fuji Film).
For immunoprecipitation, cells were lysed in RAPI buffer (10 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 600 mmol/L NP-40, 1% Triton X-100, 10% glycerol, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L sodium fluoride, 1 mmol/L sodium orthovanadate) and incubated with 20 μL protein-A sepharose beads (GE Healthcare) and 1 μg of the appropriate primary antibodies at 4 °C overnight. After washing threetimes with PBS, the samples were analyzed by immunoblotting.
For the GST pull down assay, 3 μg GST-KLF8 protein, 3 μg His-fused AR proteins, and 20 μL glutathione beads (Sigma) were incubated in 1 mL of PBS with 0.1% BSA at 4 °C for 2 h. After washing three times with PBS, the samples were analyzed by immunoblotting.
MTT cell proliferation
For the MTT assay, 2×104 cells/mL were plated in 96-well tissue culture plates (100 μL complete medium/well) and cultured at 37 °C in 5% CO2 atmosphere. At different time points, MTT reagents (DingGuo) were added (10 μL per well) and incubated at 37 °C for 4 h. To stop the reaction, 100 μL of DMSO was added, and the optical density was determined at 570 nm in a multi-well plate reader.
Xenograft nude mouse model of tumor growth
Cells were resuspended at 1×107 cells/mL, and a 0.1-mL aliquot of cell suspension was injected subcutaneously into athymic nude mice (n=10). Tumor volumes were measured at different time points by external measurements and were calculated according to the equation V=[L×W2×0.52 (V=volume, L=length and W=width).
Luciferase reporter assay
To evaluate AR-dependent transcriptional activity, a luciferase reporter assay was performed with a luciferase reporter construct (Promega). Cells were transiently transfected in triplicate with the AR-targeted luciferase reporter and pCMV-β-galactosidase (Promega) using Lipofectamine 2000 (Invitrogen). Luciferase activity was determined 48 h after transfection using the Luciferase Assay System Kit (Promega). β-Galactosidase activity was determined using the Luminescent β-gal Detection Kit II (BD Clontech) as an internal control.
qRT-PCR
An Absolutely RNA Miniprep Kit (Stratagene) was used to extract the total RNA, and the ThermoScript RT-PCR System (Invitrogen) was used for reverse transcription. SYBR-Green Master PCR Mix (Applied Biosystems) was used for PCR in triplicate. All RT2 qPCR Primer pairs were purchased from SABiosciences. The M×3000 qPCR System (Stratagene) was used for PCR and to collect data. β-Actin was used as an endogenous control. The relative quantitation value for each target gene was expressed as 2-(Ct-Cc) (Ct and Cc are the mean threshold cycle differences after normalizing to β-actin). The relative expression levels of the samples are presented in a semi-log plot.
Statistical analysis
Expression of KLF8 in PCa tumors and paired adjacent nontumoral prostate was compared using the paired Student's t-test. Kaplan-Meier survival curves were constructed, and the differences between groups were analyzed using a log rank test. P<0.05 was considered statistically significant. Statistical analysis data represent the mean±standard deviations (SD) from at least three independent experiments, each performed in triplicate, or are representative of three different experiments with similar results. All statistical tests were carried out using SPSS (Statistical Package for the Social Sciences, SPSS Inc, Chicago, IL, USA). Correlations with patient survival were also investigated by way of Cox regression using MedCalc (MedCalc Software, Mariakerke, Belgium).
Results
KLF8 is highly expressed in human PCa tissues and cell lines and is correlated with shortened survival
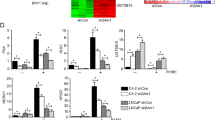
We first determined the specific expression pattern of the KLF8 protein in 133 human PCa samples by immunohistochemical analysis. Immunoreactivity to anti-KLF8 antibodies was observed in 66.15% (43/65) of low-grade PCa tissues, 92.65% (63/68) of high-grade PCa tissues and 6.82% (3/44) of adjacent normal tissues. KLF8 staining was much stronger in high-grade PCa than in low-grade PCa (Figure 1A, 1B). We then compared the expression of KLF8 with Gleason score, PSA value and clinical stage. KLF8 immunoreactivity was observed in 50% (11/22) of score 2–6 PCa tissues, 75% (45/60) of score 7 PCa tissues and 98.04% (50/51) of score 8–10 PCa tissues (Figure 1C). No KLF8 was detected in PCa tissues with a PSA value <10, but KLF8 was detected in 40% (8/20) of PCa tissues with a PSA value of 10–20, 82.2% (37/45) of PCa tissues with a PSA value of 20–30, and 100% (61/61) of PCa tissues with a PSA value >30 (Figure 1D). KLF8 was detected in 44.44% (8/18) of stage B PCa tissues, 76.36% (42/55) of stage C PCa tissues, and 96.55% (56/58) of stage D PCa tissues (Figure 1E). We then examined the expression of KLF8 in eight human PCa cell lines using anti-KLF8 antibodies. KLF8 expression was elevated in all eight PCa cell lines (Figure 1F).
Clinical significance of KLF8 expression in PCa. (A, B) Levels of KLF8 in normal tissues as well as tissues from PCa, low-grade PCa and high-grade PCa, as detected by immunostaining with an anti-KLF8 antibody. Bar=50 μm. (C) Levels of KLF8 in PCa tissues with different Gleason scores, as detected by immunostaining with an anti-KLF8 antibody. (D) Levels of KLF8 in PCa tissues with different PSA values, as detected by immunostaining with an anti-KLF8 antibody. (E) Levels of KLF8 in PCa tissues with different clinical stages, as detected by immunostaining with an anti-KLF8 antibody. (F) KLF8 expression in human PCa cell lines, as detected by immunoblotting analysis with an anti-KFL8 antibody; α-tubulin was used as a loading control. The results represent at least three separate experiments. (G) Survival rate of PCa patients based on the immunoreactivity of KLF8 in the same clinical stage (stage C).
We further evaluated whether the KLF8 immunoreactivities correlated with overall survival in 65 clinical stage C patients with PCa. KLF8 immunoreactivity was inversely correlated with overall survival (Figure 1G). These results highlight the clinical importance of KLF8 in determining the prognosis for PCa and indicate a new target for PCa therapy.
KLF8 enhances PCa cell growth in vitro and in vivo
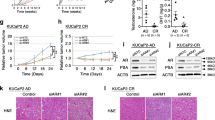
LNCap cells are androgen dependent, whereas 22Rv1 cells can serve as a model of androgen-independent prostate cancer. To investigate the biological role of KLF8 in PCa cells, we overexpressed KLF8 in human PCa LNcap cells and 22Rv1 cells (Figure 2A). We then tested the role of KLF8 in PCa cell proliferation using the MTT assay. A significant increase in the growth curve was observed, indicating that cell proliferation was enhanced in vitro in both LNcap cells and 22Rv1 cells after transfection with KLF8 (Figure 2B, 2C). We then examined whether KLF8 enhanced tumor growth in vivo. When tumor cells were injected subcutaneously into athymic nude mice, the overexpression of KLF8 resulted in dramatically increased tumor volumes compared with the vector control for both LNcap cells and 22Rv1 cells in vivo (Figure 2D, 2E). To further investigate the function of KLF8 in PCa cell proliferation and tumor growth, we used KLF8 shRNA to downregulate KLF8 in both LNCap cells and 22Rv1 cells (Figure 2F). Compared with control shRNA (Ctrl shRNA), cells treated with KLF8 shRNA grew more slowly in vitro (as determined by the MTT assay) in both LNCap cells and 22Rv1 cells (Figure 2G, 2H). Tumor volumes in mice inoculated subcutaneously with LNCap/KLF8 shRNA cells and 22Rv1/KLF8 shRNA cells were dramatically reduced compared to those in mice receiving LNCap/Ctrl shRNA and 22Rv1/Ctrl shRNA (Figure 2I, 2J). These in vitro and in vivo results demonstrate that KLF8 potently promotes PCa cell proliferation and tumor growth.
KLF8 promotes PCa cell proliferation in vitro and in vivo. (A) LNCap and 22Rv1 cells transfected with plain vector (V) or a plasmid encoding KLF8 (KLF8). Cell lysates were immunoblotted with an anti-KLF8 antibody; α-tubulin was used as a loading control. (B, C) In vitro growth of LNCap/V, 22Rv1/V (V) and LNCap/KLF8, 22Rv1/KLF8 (KLF8) cells, as measured by MTT assays. (D) Average tumor volume in athymic nude mice subcutaneously inoculated with LNCap/V (V) or LNCap/KLF8 (KLF8) cells. (E) Average tumor volume in athymic nude mice subcutaneously inoculated with 22Rv1/V (V) or 22Rv1/KLF8 (KLF8) cells. Representative tumors are shown in the right panel. (F) LNCap and 22Rv1 cells transfected with control shRNA (Ctrl shRNA) or KLF8 shRNA. KLF88 levels were detected by immunostaining with an anti-KLF8 antibody; α-tubulin was used as a loading control. (G, H) In vitro growth of LNCap/Ctrl shRNA and LNCap/KLF8 shRNA cells, as measured by MTT assays. (I) Average tumor volume in athymic nude mice subcutaneously inoculated with LNCap/Ctrl shRNA or LNCap/KLF8 shRNA cells. (J) Average tumor volume in athymic nude mice subcutaneously inoculated with 22Rv1/Ctrl shRNA or 22Rv1/KLF8 shRNA cells. bP<0.05.
KLF8 binds directly to AR
To investigate the mechanism by which KLF8 regulates PCa cell proliferation, we used a yeast two-hybrid screen to map KLF8 binding proteins. AR was identified as a positive KLF8 binding partner. We then verified the association between KLF8 and AR with a co-immunoprecipitation assay in LNCap cells, using the anti-KLF8 antibody (α-KLF8) or control rabbit IgG (rIgG) and then immunoblotting for AR or KLF8. AR co-immunoprecipitated with the anti-KLF8 antibody but not with rIgG (Figure 3A). When LNCap cells were stimulated with androgen, the binding of KLF8 and AR was enhanced (Figure 3B). We next examined whether KLF8 interacted with AR directly by using purified recombinant proteins in which a GST tag was fused to KLF8 and a His tag was fused to the N-terminus (aa1-537, His-AR-N) or C-terminus (aa538-919, His-AR-C) of AR. The C-terminus of AR but not the N-terminus bound directly to GST-KLF8 (Figure 3C, 3D). The C-terminus of AR contains a DNA binding domain (DBD) and a ligand binding domain (LBD). To determine which domain binds KLF8, we generated His-AR-DBD and His AR-LBD proteins. Using a pull down assay, we found that the DBD but not the LBD of AR bound KLF8 (Figure 3E). Taken together, our results clearly demonstrate that KLF8 binds directly to AR.
The DNA binding domain of AR binds directly with KLF8. (A) AR binding to KLF8 in LNCap cells. KLF8 was immunoprecipitated from the cell lysates, followed by immunoblotting with antibodies to KLF8 and AR. rIgG was used as an immunoprecipitation negative control. A sample loading control is shown (5% input). (B) Androgen enhances KLF8 binding with AR. (C, D, E) The C-terminus but not the N-terminus of AR binds KLF8 (C). The DNA binding domain but not the ligand binding domain of AR binds with KLF8 (D). The purified His-fused N-terminus of AR (His-AR-N), His-fused C-terminus of AR (His-AR-C), His-fused DNA binding domain of AR (His-AR-DBD), His-fused ligand binding domain of AR (His-AR-LBD) and GST-fused KLF8 (GST-KLF8) were incubated with glutathione beads as indicated. After extensive washing, the beads were immunoblotted with an anti-His antibody (α-His); GST-KLF8 was detected with an anti-GST antibody (α-GST). A sample loading control is shown (2% input).
KLF8 enhances AR target gene transcription
We then evaluated whether KLF8 affects AR transcription activity using a luciferase reporter assay. LNCap cells were co-transfected with a plasmid encoding the AR binding sites (PSA promoter) and the luciferase reporter or a system control plasmid encoding the β-galactosidase reporter gene. AR target luciferase reporter activity was robustly increased upon stimulation with androgen when KLF8 was ectopically expressed (Figure 4A). Consistently, when we knocked down KLF8 using shRNA, AR target luciferase reporter transcription was significantly decreased (Figure 4B).
KLF8 enhances the transcriptional activity of AR. (A) Overexpression of KLF8 increases the activity of the promoters of AR target genes. (B) Knockdown of KLF8 decreases AR target promoter activities. (C) The mRNA levels of the AR target genes AR and PSA are up-regulated by the overexpression of KLF8. (D) The mRNA levels of the AR target genes AR and PSA are downregulated by the knockdown of KLF8 with shRNA compared to control shRNA (KLF8 shRNA Ctrl). bP<0.05. The results represent at least three separate experiments.
To further elucidate the role of KLF8 in AR transcriptional activity, we examined the mRNA level of the AR and the AR-regulated androgen-responsive prostate specific antigen (PSA) gene. The AR gene is also an androgen-responsive gene. Indeed, ectopic expression of KLF8 triggered the expression of the AR and PSA genes in LNCap cells (Figure 4C), whereas knockdown of KLF8 attenuated their expression in LNCap cells stimulated with androgen (Figure 4D). These results clearly indicate that KLF8 is an important co-activator of AR-mediated genes.
Discussion
In this study, we demonstrated that KLF8 enhances PCa cell proliferation in vitro and in vivo and that KLF8 is a novel AR co-activator in PCa. KLF8 binds directly to AR and regulates AR-mediated transcriptional activity. More importantly, KLF8 immunoreactivity was positively correlated with increased pathological grade of PCa and inversely correlated with overall survival in patients with a diagnosis of PCa, further underscoring the clinical significance of KLF8 in the pathogenesis, prognosis, and treatment of PCa.
Androgen/AR signaling has a critical role in the growth and development of the normal prostate gland and in the proliferation and progression of prostate cancer18,19. Androgen binding to AR induces AR activation. Activated AR translocates into the nucleus, where it binds to specific androgen response elements in the promoter and enhancer regions of androgen-regulated genes and initiates the transcription of these genes20,21. Androgen deprivation is the predominant therapeutic strategy for prostate cancer and is of particular benefit to patients with low-grade tumors. However, this treatment strategy is much less effective for the long-term treatment of high-grade tumors with recurrence, a state termed castration-resistant PCa (CRPC). In CRPC, AR is usually functionally active independent of androgen22,23,24,25. A suggested mechanism for the maintenance of a functional active AR is the aberrant expression of AR co-activators, leading to increased AR activity23,26. Here, we reported that KLF8 bound AR; furthermore, the overexpression of KLF8 increased and the knockdown of KLF8 decreased AR transcription activity in both androgen-dependent LNCap cells and androgen-independent 22Rv1 cells. In particular, KLF8 was detected at much higher levels in high-grade PCa than in low-grade PCa. These results indicate that KLF8 may be an AR co-activator and contribute to high-grade PCa. The correlation between increased KLF8 expression and decreased survival rate suggests that KLF8 could be a new drug target for PCa therapy.
As a transcription factor, KLF8 can regulate the transcription of many genes. For example, KLF8 can negatively regulate the globin and E-cadherin genes through contact with the C-terminal Binding Protein (CtBP) corepressor. KLF8 can also positively regulate cyclinD1 genes by recruiting the p300 or p300/CBP associated factor (PCAF) co-activator of the histone acetylase family13,27,28. Here, we determined that KLF8 could directly bind to AR and regulate the transcription of AR-targeted genes. KLF8 could facilitate the binding of AR to chromatin or recruit other elements to bind AR. However, the mechanism by which KLF8 assists AR in regulating transcription awaits further study. In contrast to KLF8, another KLF family member, KLF4, is a tumor suppressor protein. Ectopic overexpression of KLF4 inhibits the growth and invasiveness of tumor cell lines, including prostate cancer29,30,31. The relationship between KLF8 and KLF4 requires further study.
In summary, we identified a novel AR co-activator, KLF8, that plays an important role in regulating PCa cell proliferation and tumor growth. The expression of KLF8 was also correlated with the PCa pathology stage and with the survival of PCa patients. These observations suggest that KLF8 could be a target for PCa diagnosis and therapy.
Author contribution
Yu SU and Hong-jiang HE designed the research; Hong-jiang HE, Xue-feng GU, Wan-hai XU, De-jun YANG, and Xiao-min WANG performed the research; Yu SU and Hong-jiang HE analyzed the data; and Yu SU wrote the paper.
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics. Cancer J Clin 2006; 56: 106–30.
Cheng H, Snoek R, Ghaidi F, Cox ME, Rennie PS . Short hairpin RNA knockdown of the androgen receptor attenuates ligand-independent activation and delays tumor progression. Cancer Res 2006; 66: 10613–20.
Galbraith SM, Duchesne GM . Androgens and prostate cancer: biology, pathology and hormonal therapy. Eur J Cancer 1997; 33: 545–54.
Liao X, Tang S, Thrasher JB, Griebling TL, Li B . Small-interfering RNA-induced androgen receptor silencing leads to apoptotic cell death in prostate cancer. Mol Cancer Ther 2005; 4: 505–15.
Trapman J, Brinkmann AO . The androgen receptor in prostate cancer. Pathol Res Pract 1996; 192: 752–60.
Claessens F, Verrijdt G, Schoenmakers E, Haelens A, Peeters B, Verhoeven G, et al. Selective DNA binding by the androgen receptor as a mechanism for hormone-specific gene regulation. J Steroid Biochem Mol Biol 2001; 76: 23–30.
Gelmann EP . Molecular biology of the androgen receptor. J Clin Oncol 2002; 20: 3001–15.
Heemers HV, Tindall DJ . Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev 2007; 28: 778–808.
Heinlein CA, Chang C . Androgen receptor (AR) coregulators: an overview. Endocr Rev 2002; 23: 175–200.
Feldman BJ, Feldman D . The development of androgen-independent prostate cancer. Nat Rev 2001; 1: 34–45.
Nieto M, Finn S, Loda M, Hahn WC . Prostate cancer: re-focusing on androgen receptor signaling. Int J Biochem Cell Biol 2007; 39: 1562–8.
Pearson R, Fleetwood J, Eaton S, Crossley M, Bao S . Krüppel-like transcription factors: a functional family. Int J Biochem Cell Biol 2008; 40: 1996–2001.
Zhao J, Bian ZC, Yee K, Chen BP, Chien S, Guan JL . Identification of transcription factor KLF8 as a downstream target of focal adhesion kinase in its regulation of cyclin D1 and cell cycle progression. Mol Cell 2003; 11: 1503–15.
Wang X, Zhao J . KLF8 transcription factor participates in oncogenic transformation. Oncogene 2007; 26: 456–61.
Wang X, Urvalek AM, Liu J, Zhao J . Activation of KLF8 transcription by focal adhesion kinase in human ovarian epithelial and cancer cells. J Biol Chem 2008; 283: 13934–42.
Li JC, Yang XR, Sun HX, Xu Y, Zhou J, Qiu SJ, et al. Up-regulation of Krüppel-like factor 8 promotes tumor invasion and indicates poor prognosis for hepatocellular carcinoma. Gastroenterology 2010; 139: 2146–57.
Wang X, Zheng M, Liu G, Xia W, McKeown-Longo PJ, Hung MC, et al. Krüppel-like factor 8 induces epithelial to mesenchymal transition and epithelial cell invasion. Cancer Res 2007; 67: 7184–93.
Debes JD, Tindall DJ . Mechanisms of androgen-refractory prostate cancer. N Engl J Med 2004; 351: 1488–90.
Lamont KR, Tindall DJ . Minireview: alternative activation pathways for the androgen receptor in prostate cancer. Mol Endocrinol 2011; 25: 897–907.
Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M . Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 2000; 103: 843–52.
Kang Z, Pirskanen A, Janne OA, Palvimo JJ . Involvement of proteasome in the dynamic assembly of the androgen receptor transcription complex. J Biol Chem 2002; 277: 48366–71.
Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T . Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res 2001; 61: 3550–5.
Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med 2004; 10: 33–9.
Mohler JL, Gregory CW, Ford OH 3rd, Kim D, Weaver CM, Petrusz P, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res 2004; 10: 440–8.
Taplin ME, Balk SP . Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem 2004; 91: 483–90.
Chmelar R, Buchanan G, Need EF, Tilley W, Greenberg NM . Androgen receptor coregulators and their involvement in the development and progression of prostate cancer. Int J Cancer 2007; 120: 719–33.
Van Vliet J, Turner J, Crossley M . Human Kruppel-like factor 8: a CACCC-box binding protein that associates with CtBp and represses transcription. Nucleic Acids Res 2000; 28: 1955–62.
Urvalek AM, Wang X, Lu H, Zhao J . KLF8 recruits the p300 and PCAF co-activators to its amino terminal activation domain to activate transcription. Cell Cycle 2010; 9: 601–11.
Wang J, Place RF, Huang V, Wang X, Noonan EJ, Magyar CE, et al. Prognostic value and function of KLF4 in prostate cancer: RNAa and vector-mediated overexpression identify KLF4 as an inhibitor of tumor cell growth and migration. Cancer Res 2010; 70: 10182–91.
Wei D, Gong W, Kanai M, Schlunk C, Wang L, Yao JC, et al. Drastic down-regulation of Kruppel-like factor 4 expression is critical in human gastric cancer development and progression. Cancer Res 2005; 65: 2746–54.
Yori JL, Seachrist DD, Johnson E, Lozada KL, Abdul-karim FW, Chodosh LA, et al. Kruppel-like factor 4 inhibits tumorigenic progression and metastasis in a mouse model of breast cancer. Neoplasia 2011; 13: 601–10.
Acknowledgements
This study was supported by the Heilongjiang Province Health Department Fund (No 2010-135).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, Hj., Gu, Xf., Xu, Wh. et al. Krüppel-like factor 8 is a novel androgen receptor co-activator in human prostate cancer. Acta Pharmacol Sin 34, 282–288 (2013). https://doi.org/10.1038/aps.2012.130
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2012.130
Keywords
This article is cited by
-
Role of Krüppel-like factors in cancer stem cells
Journal of Physiology and Biochemistry (2015)