Abstract
Aim:
To investigate the pharmacokinetics of imatinib in Chinese chronic myelogenous leukemia (CML) patients.
Methods:
Fourty-six naïve Chinese CML patients treated with imatinib (400 and 600 mg daily, n=36 and 10, respectively) were recruited. The correlations of imatinib (400 mg) trough plasma concentrations (Cmins) with the patients' characteristics and responses were analyzed.
Results:
The overall mean (±SD, CV%) steady-state Cmins for imatinib at 400 mg (n=36) and 600 mg (n=10) daily was 1325.61 ng/mL (±583.53 ng/mL; 44%) and 1550.90 ng/mL (±462.63 ng/mL; 30%), respectively, and no statistically significant differences were found between them (P=0.267). At 400 mg daily, female patients had significantly higher Cmins than the male patients (P=0.048), and molecular responses were not correlated with imatinib Cmins, but they were correlated with time elapsed before imatinib therapy.
Conclusion:
The results suggest that Chinese CML patients have higher imatinib Cmins than their Caucasian counterparts and that the optimal initial imatinib dose for them requires further investigation.
Similar content being viewed by others
Introduction
Chronic myelogenous leukemia (CML) is a clonal myeloproliferative disorder of the primitive hematopoietic stem cell. It is characterized by the hybrid oncogene BCR-ABL, which codes for the novel BCR-ABL oncoprotein. This oncoprotein has uncontrolled kinase activity that triggers the excessive proliferation and reduced apoptosis of CML cells1, 2. Imatinib mesylate (Gleevec, Glivec), a potent and specific inhibitor of the BCR-ABL tyrosine kinase, is now the first-line treatment for CML. Imatinib has significantly improved the prognosis of CML3. The International Randomized Study of Interferon and STI571 (IRIS) trial found an estimated cumulative complete cytogenetic response rate (CCyR) and overall survival of 87% and 89%, respectively, among 553 patients who received first-line imatinib for five years4.
Despite the impressive rate of response, some CML patients show primary resistance to imatinib or relapse after an initial response (acquired resistance)5. Resistance to imatinib caused by BCR-ABL gene amplification or increased levels of mRNA can be overcome by increasing the imatinib dose6, 7, 8. More recently, it has been suggested that variations in imatinib trough plasma concentrations (Cmins) could affect cytogenetic and molecular responses in CML. Picard et al9 reported that imatinib Cmins were associated with both CCyR and major molecular response (MMR) to standard-dose imatinib (400 mg daily) in CML, with a plasma threshold of 1002 ng/mL in vivo. A subanalysis of the IRIS trial indicated that patients with high imatinib exposure had better rates of CCyR and MMR10. Singh et al11 also reported that the mean Cmins of imatinib responders were significantly higher than those of non-responders. However, it was also reported that there was no correlation between mean Cmins and CCyR or MMR12.
The pharmacokinetics of imatinib has been extensively studied in Caucasian CML patients, and very recently Sakai et al13 found that a lower dose of imatinib could maintain enough imatinib Cmins and provided excellent results for the treatment of CML in a Japanese registry. However, few studies have been reported in a Chinese population. In the present study, we measured steady-state Cmins in CML patients who received imatinib for at least 12 months (400 mg or 600 mg daily) and described the pharmacokinetic features of Chinese CML patients. We assessed how Chinese patients' characteristics correlated with imatinib Cmins and how imatinib Cmins correlated with complete molecular response (CMR). Finally, a rough comparison between Chinese patients and the patients of the IRIS was performed to gather clues to the optimal initial imatinib dose for Chinese CML patients.
Materials and methods
Patients
The study was conducted in accordance with the Declaration of Helsinki, and all patients provided written informed consent. Ethics approval was obtained from the Independent Ethics Committee of Union Hospital, Tongji College, Huazhong University of Science and Technology. All CML out-patients who visited the hospital between the 1st and 31st of October 2008 were considered for our study. Inclusion criteria were a) first chronic-phase or accelerated-phase CML treated orally with standard-dose imatinib (400 mg and 600 mg once daily for chronic-phase and accelerated-phase CML, respectively) and b) imatinib treatment duration ≥12 months. In total, 160 CML patients were considered for participation in the study. Exclusion criteria were a) blast crisis before or during imatinib therapy, b) blood collection performed out of the trough concentration time limits, c) poor compliance with treatment, or d) identification of gene mutation(s) in the kinase domain of BCR-ABL.
Of those considered, 114 patients were excluded because of inadequate blood collection performed out of time limits, use of a nonstandard drug dose, recognized poor compliance with therapy (one patient) or progression to blast phase (two patients). Ultimately, 46 patients with CML were included for investigation: 36 and 10 were treated with 400 mg and 600 mg imatinib daily, respectively. All patients were in chronic phase, except two patients treated with 600 mg IM daily in accelerated phase. There were 30 male and 16 female patients. Median age was 38 years (range, 17–79 years). The mean body weight was 61.72±9.67 (SD) kg (median, 60.00 kg; range, 40.0-85.0 kg), and the mean body surface area (BSA) was 1.66±0.14 m2 (median, 1.64 m2; range, 1.36–1.97 m2). The median duration of imatinib therapy at the time of Cmins testing was 28.57 months (range, 12–74 months).
Sample collection and disease response analysis
Bone marrow was aspirated from the posterior iliac crest of the patients for cytogenetic evaluation. This was performed at diagnosis and every 6 months for the first 2 years, and then yearly thereafter, unless clinical or laboratory features suggested disease progression. CCyR was defined as all 20 bone marrow metaphases analyzed being Ph-14. However, because of frequent failure of bone marrow aspiration in CML patients receiving imatinib therapy, collection of bone marrow was severely disrupted. Thus, owing to limited data collection, the correlation between Cmins and achievement of CCyR was not shown and analyzed.
To assess molecular responses, we extracted total RNA from peripheral blood cells and quantified BCR-ABL transcript concentrations using real-time quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR)15, according to recently proposed recommendations for harmonization of results16. CMR was defined as undetectable BCR-ABL transcripts by RQ-PCR. The most recent RQ-PCR results were used to determine molecular response.
Sample collection and pharmacokinetic analysis
For imatinib plasma quantification, blood samples were collected 21–27 h after the last drug administration but before the next administration. Plasma was isolated by centrifugation and frozen plasma samples (−20 °C) were sent to WuXi PharmaTech (Shanghai, China) to be analyzed using liquid chromatography/tandem mass spectrometry (LC-MS/MS). The limit of quantification of imatinib was 2.00 ng/mL (range 2.00–10 000 ng/mL).
Statistical analysis
The Student's t-test or Wilcoxon signed-rank test was used to compare means between the two groups. The chi-square test or Fisher's exact text was used for comparisons of proportions. Pearson correlation or Spearman's correlation analysis of Cmins and patients' characteristics (including age, weight, BSA, time elapsed before imatinib therapy and duration of therapy) was performed in patients treated with 400 mg IM daily.
Results
Imatinib plasma trough concentrations of Chinese CML patients receiving the standard dose
Thirty-six patients in first chronic phase—23 males and 13 females—were treated with 400 mg imatinib daily. Their mean imatinib Cmin was 1325.61±583.53 ng/mL, with 44% interpatient variability. For the 23 males, the mean body weight was 64.70±10.81 kg (median, 63.00 kg; range, 40.0–85.0 kg), and the mean body surface area (BSA) was 1.72±0.14 m2 (median, 1.70 m2; range, 1.40–1.97 m2). For the 13 females, the mean body weight was 56.39±6.68 kg (median, 59.0 kg; range, 46.0–65.0 kg), and the mean BSA was 1.55±0.11 m2 (median, 1.61 m2; range, 1.36–1.68m2). For all patients, the median age was 38 years (range, 17–79 years), the mean elapsed time before imatinib therapy was 24.75 months (range, 1–84 months), and the median duration of imatinib therapy at the time of Cmins testing was 28.97 months (range, 12–74 months). Table 1 shows the clinical characteristics of the 36 patients who were given 400 mg IM daily. Only body weight (P=0.017) and BSA (P=0.001) were significantly higher in males than in females.
The mean Cmin of the 10 patients treated with 600 mg im daily was 1550.90±462.63 ng/mL, with 30% interpatient variability.
Correlation of imatinib plasma trough concentrations with patients' characteristics and molecular responses
After Pearson or Spearman's correlation analysis, we found no significant correlation of imatinib Cmins with age, time elapsed before imatinib therapy and duration of imatinib therapy. However, female patients had significantly higher imatinib Cmins than male patients (1579.69±510.50 ng/mL vs 1182.00±582.97 ng/mL, P=0.048). Because of the limited number of patients, the same correlation analysis was not performed in patients treated with 600 mg imatinib daily. No significant differences were found between patients treated with 400 mg (n=36) and 600 mg (n=10) imatinib daily (1325.61±583.53 ng/mL vs 1550.90±462.63 ng/mL, P=0.267).
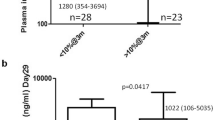
As shown in Table 2, patients who received imatinib therapy earlier had a significantly higher likelihood of achieving CMR (time elapsed before imatinib therapy: CMR vs no CMR, 20.57±17.21 months vs 39.38±23.11 months, P=0.016). Although the female patients have higher imatinib Cmins than the males, they did not have a significantly higher rate of CMR (77% vs 78%, P=1.000). There was no statistically significant difference in imatinib Cmins between patients in the CMR group and the no-CMR group (CMR vs no CMR, 1263.39±532.55 ng/mL vs 1543.38±734.11 ng/mL, P=0.237). Interestingly, the imatinib Cmins were higher in the no-CMR group, but this result was not statistically significant (Figure 1).
Steady-state imatinib trough levels in chronic-phase CML patients who did and did not achieve CMR. The mean plasma imatinib trough levels were 1263.39±532.55 ng/mL and 1543.38±734.11 ng/mL for patients who did (n=28) and did not (n=8) achieve CMR, respectively (P=0.237). The top and bottom walls of each Cmin (trough level) plot box represent the 75th and 25th percentiles, respectively.
Comparison of imatinib Cmins between Chinese patients and patients in the IRIS study
Patients in the IRIS study were all treated with 400 mg daily. The mean Cmins of male patients, female patients and all patients were 921±531, 1078±515, and 979±530 ng/mL, respectively10. As shown in Table 3, the mean imatinib Cmin of the patients in the present study seem to be higher than that of the IRIS study (1325.61±583.53 ng/mL vs 979±530 ng/mL), and the patients' body weight and BSA also seem to be significantly different between the two studies.
Discussion
The pharmacokinetics of imatinib has been extensively studied in Caucasian CML patients17, 18, 19, 20, 21. However, few studies have been reported on naïve Chinese. Recently, Wang et al22 reported the effects of imatinib on the pharmacokinetics of metoprolol, a CYP2D6 substrate, in Chinese patients with CML. Although the authors paid more attention to the pharmacokinetics of metoprolol than imatinib, the study suggested significant differences between Caucasian and naïve Chinese patients treated with 400 mg im twice daily. It suggested that the plasma Cmax and AUC values of imatinib observed in Chinese patients—4245 ng/mL and 39 041 ng·mL−1·h−1, respectively—were approximately 15% higher than those in Caucasian patients receiving the same dose (800 mg daily) in the phase I study, 3702±1434 ng/mL and 34 200±14 900 ng·mL−1·h−1, respectively. The estimated effective half-life was approximately 25 h, somewhat longer than the approximately 20 h for Caucasian patients. The clearance in Chinese patients (11.3 L/h) appeared to be in the lower end of the values reported for Caucasian patients, between 10 and 14 L/h. Our data provided the mean imatinib Cmins for Chinese CML patients treated with 400 mg or 600 mg im daily. We also found that female CML patients treated with 400 mg imatinib daily had significantly higher Cmins than males (P=0.048), a disparity that may originate in differences in body weight and BSA, as reported10; however, no correlations were found in the other characteristics of the patients with imatinib Cmins.
In the correlation analysis of disease responses with patients' characteristics, molecular responses to 400 mg imatinib daily are correlated only with time elapsed before imatinib therapy. It suggests that taking imatinib as soon as possible helps to achieve CMR. Our data agree with the conclusion that imatinib Cmins are not correlated with molecular response12. An odd result was that the mean imatinib Cmins in our patients were a littler higher in the no-CMR group than in the CMR group (P=0.237). Picard et al9 concluded that imatinib plasma trough concentrations were correlated with both cytogenetic and molecular responses to standard-dose imatinib in CML, and the plasma threshold for Cmins was set at 1002 ng/mL because it provided the best discrimination potential for achieving MMR. This conclusion was supported by other studies10, 11. The inability to find a significant difference between patients in the CMR group and the no-CMR group is probably due to the fact that all of our patients had exceeded the plasma threshold of 1002 ng/mL, and this can in part explain why the patients treated with 600 mg imatinib daily didn't have significantly higher Cmins than patients treated with 400 mg daily (P=0.267).
In the rough comparison of imatinib Cmins between Chinese patients and IRIS patients, similar results were found in Chinese and Caucasian patients, such as the large interpatient variability in imatinib Cmins and higher imatinib Cmins in female patients due to lower body weight and BSA. As other reports have explained9, 10, 11, the reason for the interpatient variability in imatinib Cmins may be the intrinsic variability of the CYP system and drug efflux/influx transporters, such as P-glycoprotein (P-gp or ABCB1), breast cancer resistance protein (BCRP or ABCG2), multidrug resistance protein 1 (MRP1 or ABCC1) and human organic cation transporter-1 (OCT1). The difference in imatinib Cmins between Chinese and Caucasians patients was unavoidable. The difference between the results of the present study and IRIS seems to be due to different body weight and BSA. Our findings, which are in agreement with the only finding22 in Chinese CML patients, suggest that imatinib exposure in Chinese CML patients was higher than in Caucasian patients.
It was reported that dose intensity was critical to optimize response in CML23, 24, 25, 26, 27. Based on higher drug exposure in Chinese patients and dose-proportional imatinib exposure18, 19, a rational conjecture is that Chinese patients would have a better disease response. We can infer that the patients in our studies had have better disease responses, even though CMR was not monitored in the IRIS study, in which BCR-ABL transcripts in the blood samples were measured only at 1 and 4 years in 124 patients who had a CCyR. After 1 year, levels of BCR-ABL transcripts had fallen by at least 3 log in 66 of 124 patients (53%); after 4 years, levels had fallen in 99 of 124 patients (80%)4. In our study, after a mean follow-up of 28.97 months (range 12–84 months), no BCR-ABL transcripts were detected in 78% of the patients treated with 400 mg imatinib daily regardless of whether they acquired CCyR. However, because of the small number of patients, this conclusion requires further investigation. Another reason that we could not report the exact CMR rate of the patients in our hospital was that some patients with suboptimal response received a dose escalation from 500 mg to 800 mg daily.
Higher drug exposure also leads to more adverse events. As we previously reported, in CML patients treated with a standard dose of imatinib, grade 3 leukocytopenia occurred in 16.0% of the patients and grade 3 thrombocytopenia occurred in 18.0% of the patients, respectively28. In Japanese studies, grade 3 or 4 neutropenia and/or leukocytopenia occurred in 33.3% of patients, and grade 3 thrombocytopenia occurred in 12.8% of patients29. Given Asians' lower body weight and BSA, both Korean and Japanese doctors doubted whether 400 mg daily of imatinib was an optimal initial dose for Asian patients and proposed a lower dose30, 31, 32, 33, 34. We found that the imatinib plasma trough concentration of Chinese patients who received 400 mg imatinib daily was higher than that of Caucasian patients, which provides convincing proof that Asian patients with lower body weight or BSA need a lower dose of imatinib than Caucasian CML patients.
In summary, our work provides a study of the pharmacokinetics of imatinib in Chinese CML and suggests that receiving imatinib treatment as soon as possible is helpful to achieve CMR. Imatinib Cmins are not correlated with molecular response in Chinese CML patients in chronic phase treated with 400 mg imatinib daily. Chinese CML patients seem to have higher imatinib Cmins than Caucasian CML patients, which probably results in a better disease response. However, it causes more severe adverse events, and so the optimal initial imatinib dose for Chinese patients requires further investigation.
Author contribution
Yong YOU, Ping ZOU, and Zhi-chao CHEN designed the research; Qiu-bai LI, Chao CHEN, and Hong-xiang WANG performed the research; Chao CHEN and Yan-lin WU analyzed the data; Qiu-bai LI and Chao CHEN wrote the paper.
References
Melo JV, Deininger MW . Biology of chronic myelogenous leukemia–signaling pathways of initiation and transformation. Hematol Oncol Clin North Am 2004; 18: 545–68.
Lugo TG, Pendergast AM, Muller AJ, Witte ON . Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science 1990; 247: 1079–82.
Druker BJ . Translation of the Philadelphia chromosome into therapy for CML. Blood 2008; 112: 4808–17.
Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. New Engl J Med 2006; 355: 2408–17.
Apperley JF . Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol 2007; 8: 1018–29.
Volpe G, Panuzzo C, Ulisciani S, Cilloni D . Imatinib resistance in CML. Cancer Lett 2009; 274: 1–9.
Crossman LC, Druker BJ, Deininger MW, Pirmohamed M, Wang L, Clark RE . hOCT1 and resistance to imatinib. Blood 2005; 106: 1133–4.
White DL, Saunders VA, Dang P, Engler J, Venables A, Zrim S, et al. Most CML patients who have a suboptimal response to imatinib have low OCT-1 activity: higher doses of imatinib may overcome the negative impact of low OCT-1 activity. Blood 2007; 110: 4064–72.
Picard S, Titier K, Etienne G, Teilhet E, Ducint D, Bernard MA, et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood 2007; 109: 3496–9.
Larson RA, Druker BJ, Guilhot F, O'Brien SG, Riviere GJ, Krahnke T, et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood 2008; 111: 4022–8.
Singh N, Kumar L, Meena R, Velpandian T . Drug monitoring of imatinib levels in patients undergoing therapy for chronic myeloid leukaemia: comparing plasma levels of responders and non-responders. Eur J Clin Pharmacol 2009; 65: 545–9.
Forrest DL, Trainor S, Brinkman RR, Barnett MJ, Hogge DE, Nevill TJ, et al. Cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia are correlated with Sokal risk scores and duration of therapy but not trough imatinib plasma levels. Leuk Res 2009; 33: 271–5.
Sakai M, Miyazaki Y, Matsuo E, Moriuchi Y, Hata T, Fukushima T, et al. Long-term efficacy of imatinib in a practical setting is correlated with imatinib trough concentration that is influenced by body size: a report by the Nagasaki CML Study Group. Int J Hematol 2009; 89: 319–25.
O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003; 348: 994–1004.
Kreuzer KA, Lass U, Bohn A, Landt O, Schmidt CA . LightCycler technology for the quantitation of bcr/abl fusion transcripts. Cancer Res 1999; 59: 3171–4.
Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood 2006; 108: 28–37.
Rousselot P, Huguet F, Rea D, Legros L, Cayuela JM, Maarek O, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood 2007; 109: 58–60.
Peng B, Hayes M, Resta D, Racine-Poon A, Druker BJ, Talpaz M, et al. Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol 2004; 22: 935–42.
Peng B, Lloyd P, Schran H . Clinical pharmacokinetics of imatinib. Clin Pharmacokinet 2005; 44: 879–94.
Schmidli H, Peng B, Riviere GJ, Capdeville R, Hensley M, Gathmann I, et al. Population pharmacokinetics of imatinib mesylate in patients with chronicphase chronic myeloid leukaemia: results of a phase III study. Br J Clin Pharmacol 2005; 60: 35–44.
Gschwind HP, Pfaar U, Waldmeier F, Zollinger M, Sayer C, Zbinden P, et al. Metabolism and disposition of imatinib mesylate in healthy volunteers. Drug Metab Dispos 2005; 33: 1503–12.
Wang Y, Zhou L, Dutreix C, Leroy E, Yin Q, Sethuraman V, et al. Effects of imatinib (Glivec) on the pharmacokinetics of metoprolol, a CYP2D6 substrate, in Chinese patients with chronic myelogenous leukaemia. Br J Clin Pharmacol 2008; 65: 885–92.
Cortes J, Giles F, O'Brien S, Thomas D, Garcia-Manero G, Rios MB, et al. Result of high-dose imatinib mesylate in patients with Philadelphia chromosome-positive chronic myeloid leukemia after failure of interferon-α. Blood 2003; 102: 83–6.
Kantarjian HM, Talpaz M, O'Brien S, Giles F, Garcia-Manero G, Faderl S, et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood 2003; 101: 473–5.
Kantarjian H, Talpaz M, O'Brien S, Garcia-Manero G, Verstovsek S, Giles F, et al. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood 2004; 103: 2873–8.
Deenik W, van der Holt B, Verhoef GE, Smit WM, Kersten MJ, Kluin-Nelemans HC, et al. Dose finding study of imatinib in combination with intravenous cytarabine: feasibility in newly diagnosed patients with chronic myeloid leukemia. Blood 2008; 111: 2581–8.
Hughes TP, Branford S, White DL, Reynolds J, Koelmeyer R, Seymour JF, et al. Impact of early dose intensity on cytogenetic and molecular responses in chronic-phase CML patients receiving 600 mg/day of imatinib as initial therapy. Blood 2008; 112: 3965–73.
Chen ZC, You Y, Zhu XM, Li QB, Li WM, Zou P . A clinical study of treating 120 cases of adult chronic myelocytic leukemia with imatinib mesylate. Zhonghua Nei Ke Za Zhi 2007; 46: 1003–6.
Morishima Y, Ogura M, Nishimura M, Yazaki F, Bessho M, Mizoguchi H, et al. Efficacy and safety of imatinib mesylate for patients in the first chronic phase of chronic myeloid leukemia: results of a Japanese phase II clinical study. Int J Hematol 2004; 80: 261–6.
Miyazawa K, Nishimaki J, Katagiri T, Sashida G, Shoji N, Kawakubo K, et al. Thrombocytopenia induced by imatinib mesylate (Glivec) in patients with chronic myelogenous leukemia: is 400 mg daily of imatinib mesylate an optimal starting dose for Japanese patients? Int J Hematol 2003; 77: 93–5.
Horikoshi A, Takei K, Sawada S . Effects of lower dose of imatinib to CML patients. Leuk Res 2003; 27: 1167.
Horikoshi A, Takei K, Sawada S . Relationship between the daily dose of imatinib per square meter, and its plasma concentration in patients with chronic-phase chronic myeloid leukemia (CML). Leuk Res 2007; 31: 574–5.
Park SJ, Choi IK, Seo HY, Sung HJ, Park KH, Kim SJ, et al. Reduced dose of imatinib for patients with chronic myeloid leukemia and low body surface area. Acta Haematol 2007; 118: 219–21.
Kobayashi S, Kimura F, Kobayashi A, Sato K, Motoyoshi K . Efficacy of low-dose imatinib in chronic-phase chronic myelogenous leukemia patients. Acta Haematol 2009; 88: 311–5.
Acknowledgements
We thank Qing LI for helping collect patients' disease history, Dr Jian-qiong LIU for guidance with data analysis, and Wuxi PharmaTech for imatinib plasma trough concentration analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Qb., Chen, C., Chen, Zc. et al. Imatinib plasma trough concentration and its correlation with characteristics and response in Chinese CML patients. Acta Pharmacol Sin 31, 999–1004 (2010). https://doi.org/10.1038/aps.2010.79
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.79
Keywords
This article is cited by
-
Pharmacokinetics of Imatinib Mesylate and Development of Limited Sampling Strategies for Estimating the Area under the Concentration–Time Curve of Imatinib Mesylate in Palestinian Patients with Chronic Myeloid Leukemia
European Journal of Drug Metabolism and Pharmacokinetics (2023)
-
Early BCR-ABL1 decline in imatinib-treated patients with chronic myeloid leukemia: results from a multicenter study of the Chinese CML alliance
Blood Cancer Journal (2018)