Abstract
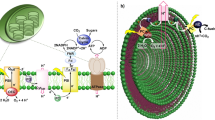
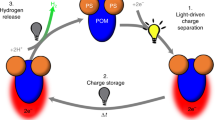
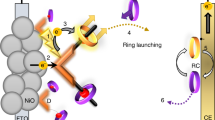
Solar energy is being used for power generation, but also attracts increasing interest as a renewable energy source for the photocatalytic production of useful chemicals. Simple systems based on vesicles with transmembrane redox mediators have been used to transform photon energy into long-lived, membrane-separated photoredox products1,2,3,4,5,6. However, these systems are not suitable for high-throughput applications because the transmembrane electron carriers are oxidized inside the vesicle into charged species that are no longer able to readily traverse the membrane bilayer. This leads to continuous trapping of these carriers during photolysis and, ultimately, to the termination of the redox reaction due to accumulation of the available carriers within the vesicle interior. Living cells circumvent this problem by using quinones to simultaneously transport electrons and protons, thus allowing the carrier to remain neutral in its reduced and oxidized states and so retain the ability to undergo transmembrane diffusion throughout the redox cycle. But the incorporation of quinones into artificial systems is not practical because of their susceptibility to oxidative degradation and slow transmembrane diffusion7. Here we describe an alternative mechanism for rapid electroneutral charge transport across vesicle membranes: we use pyrylium cations as the electron carrier, which undergo reversible ring-opening hydrolysis to form neutral diketones after deposition of the electron inside the vesicle. As the pyrylium cations are also the primary acceptors for the photoproduced electrons, our approach greatly simplifies the design of vesicle-based photocatalytic devices.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hurst,J. K. in Kinetics and Catalysis in Microheterogeneous Systems (eds Grätzel, M. & Kalyanasunderam, K.) 183–226 (Surfactant Science Series, Vol. 38, Marcel Dekker, New York, 1991).
Lymar,S. V., Parmon,V. N. & Zamaraev,K. I. in Photoinduced Electron Transfer III (ed. Mattay, J.) 1–66 (Topics in Current Chemistry, Vol. 159, Springer, Berlin, 1991).
Robinson,J. N. & Cole-Hamilton,D. J. Electron transfer across vesicle bilayers. Chem. Soc. Rev. 20, 49–94 (1991).
Lymar,S. V., Khairutdinov,R. F., Soldatenkova,V. A. & Hurst,J. K. N-Alkylpyridinium-mediated photoinduced charge separation across dihexadecyl phosphate vesicle membranes. J. Phys. Chem. B 102, 2811–2819 (1998).
Lymar,S. V. & Hurst,J. K. Electrogenic and electroneutral pathways for methyl viologen-mediated transmembrane oxidation-reduction across dihexadecylphosphate vesicular membranes. J. Phys. Chem. 98, 989–996 (1994).
Hammarstrom,L., Almgren,M. & Norrby,T. Transmembrane electron transfer mediated by a viologen: a mechanism involving diffusion of doubly reduced viologen formed by disproportionation of viologen radical. J. Phys. Chem. 96, 5017–5024 (1992).
Futami,A., Hurt,E. & Hauska,G. Vectorial redox reactions of physiological quinones. 1. Requirements of a minimal length of the isoprenoid side chain. Biochim. Biophys. Acta 547, 583–596 (1979).
Berson,J. A. Ring-chain tautomerism of pyrylium pseudo-bases. J. Am. Chem. Soc. 74, 358–360 (1952).
Williams,A. The hydrolysis of pyrylium salts. Kinetic evidence for hemiacetal intermediates. J. Am. Chem. Soc. 93, 2733–2737 (1971).
Tricot,Y.-M. et al. Dihexadecylphosphate vesicle dispersions—preparation, physical properties, and interactions with cationic components used in the solar photolysis of water. J. Colloid Interface Sci. 97, 380–391 (1984).
Kalyanasundaram,K. & Neumann-Spallart,M. Photophysical and redox properties of water-soluble porphyrins in aqueous media. J. Phys. Chem. 86, 5163–5169 (1982).
Wintgens,V., Pouliquen,J., Kossanyi,J. & Heitz,M. Reduction of pyrylium salts: study by ESR and uv-visible spectroscopy of the reversible dimerization of pyranyl radical. Nouv. J. Chem. 10, 345–350 (1986).
Neta,P. One-electron transfer reactions involving zinc and cobalt porphyrins in aqueous solutions. J. Phys. Chem. 85, 3678–3684 (1981).
Kuriyama,Y., Arai,T., Sakuragi,H. & Tokumaru,K. Effects of sensitizer spin multiplicity on electron transfer-initiated isomerization of cis-stilbene. Chem. Lett. 1193–1196 (1988).
Dickinson,T. & Wynne-Jones,W. F. K. Mechanism of Kolbe's electrosynthesis. Trans. Faraday Soc. 58, 382–387 (1962).
Walter,A. & Gutknecht,J. Permeability of small nonelectrolytes through lipid bilayer membranes. J. Membr. Biol. 90, 207–217 (1986).
Farina,R. & Wilkins,R. G. Electron transfer rate studies of a number of cobalt(II)-cobalt(III) systems. Inorg. Chem. 7, 514–518 (1968).
Surdhar,P. S. & Armstrong,D. A. Reduction potentials and exchange reactions of thiyl radicals and disulfide anion radicals. J. Phys. Chem. 91, 6532–6537 (1987).
Acknowledgements
This work was supported by the Office of Basic Energy Sciences, US Department of Energy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khairutdinov, R., Hurst, J. Cyclic transmembrane charge transport by pyrylium ions in a vesicle-based photocatalytic system. Nature 402, 509–511 (1999). https://doi.org/10.1038/990064
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/990064
This article is cited by
-
Light-activated calcium gradients
Nature Materials (2002)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.