Abstract
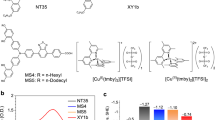
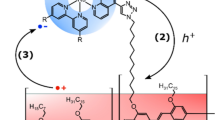
Molecular photoelectrochemical devices are hampered by electron–hole recombination after photoinduced electron transfer, causing losses in power conversion efficiency. Inspired by natural photosynthesis, we demonstrate the use of supramolecular machinery as a strategy to inhibit recombination through an organization of molecular components that enables unbinding of the final electron acceptor upon reduction. We show that preorganization of a macrocyclic electron acceptor to a dye yields a pseudorotaxane that undergoes a fast (completed within ~50 ps) ‘ring-launching’ event upon electron transfer from the dye to the macrocycle, releasing the anionic macrocycle and thus reducing charge recombination. Implementing this system into p-type dye-sensitized solar cells yielded a 16-fold and 5-fold increase in power conversion efficiency compared to devices based on the two control dyes that are unable to facilitate pseudorotaxane formation. The active repulsion of the anionic macrocycle with concomitant reformation of a neutral pseudorotaxane complex circumvents recombination at both the semiconductor–electrolyte and semiconductor–dye interfaces, enabling a threefold enhancement in hole lifetime.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All processed data that support the findings of this study are available within the article and its Supplementary Information (synthesis of compounds and characterization, device fabrication and characterization, and femtosecond TA). All raw data that support the findings of this study have been deposited at the FigShare repository https://doi.org/10.6084/m9.figshare.20508852.v1. Source data are provided with this paper.
References
Dau, H. & Zaharieva, I. Principles, efficiency, and blueprint character of solar-energy conversion in photosynthetic water oxidation. Acc. Chem. Res. 42, 1861–1870 (2009).
Zhang, B. & Sun, L. Artificial photosynthesis: opportunities and challenges of molecular catalysts. Chem. Soc. Rev. 48, 2216–2264 (2019).
Croce, R. & van Amerongen, H. Natural strategies for photosynthetic light harvesting. Nat. Chem. Biol. 10, 492–501 (2014).
Van Eerden, F. J., Melo, M. N., Frederix, P. W. J. M., Periole, X. & Marrink, S. J. Exchange pathways of plastoquinone and plastoquinol in the photosystem II complex. Nat. Commun. 8, 15214 (2017).
Kulik, N., Kutý, M. & Řeha, D. The study of conformational changes in photosystem II during a charge separation. J. Mol. Model. 26, 26–75 (2020).
O’Regan, B. & Gratzel, M. A low-cost, high-efficiency solar-cell based on dye-sensitized colloidal TiO2 films. Nature 353, 737–740 (1991).
Kakiage, K. et al. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 51, 15894–15897 (2015).
Perera, I. R. et al. Application of the tris(acetylacetonato)iron(III)/(II) redox couple in p-type dye-sensitized solar cells. Angew. Chem. Int. Ed. 54, 3758–3762 (2015).
Mori, S. et al. Charge-transfer processes in dye-sensitized NiO solar cells. J. Phys. Chem. C 112, 16134–16139 (2008).
Nakade, S. et al. Influence of TiO2 nanoparticle size on electron diffusion and recombination in dye-sensitized TiO2 solar cells. J. Phys. Chem. B 107, 8607–8611 (2003).
Morandeira, A., Boschloo, G., Hagfeldt, A. & Hammarström, L. Photoinduced ultrafast dynamics of coumarin 343 sensitized p-type-nanostructured NiO films. J. Phys. Chem. B 109, 19403–19410 (2005).
Daeneke, T. et al. Dominating energy losses in NiO p-type dye-sensitized solar cells. Adv. Energy Mater. 5, 1401387 (2015).
Parlane, F. G. L. et al. Spectroscopic detection of halogen bonding resolves dye regeneration in the dye-sensitized solar cell. Nat. Commun. 8, 1761 (2017).
Uemura, Y., Murakami, T. N. & Koumura, N. Crown ether-substituted carbazole dye for dye-sensitized solar cells: controlling the local ion concentration at the TiO2/dye/electrolyte interface. J. Phys. Chem. C 118, 16749–16759 (2014).
Wood, C. J. et al. Red-absorbing cationic acceptor dyes for photocathodes in tandem solar cells. J. Phys. Chem. C 118, 16536–16546 (2014).
Bouwens, T., Mathew, S. & Reek, J. N. H. p-Type dye-sensitized solar cells based on pseudorotaxane mediated charge-transfer. Faraday Discuss. 215, 393–406 (2019).
Cheng, C. et al. An artificial molecular pump. Nat. Nanotechnol. 10, 547–553 (2015).
Cai, K. et al. Molecular-pump-enabled synthesis of a daisy chain polymer. J. Am. Chem. Soc. 142, 10308–10313 (2020).
Simpson, C. D. et al. Nanosized molecular propellers by cyclodehydrogenation of polyphenylene dendrimers. J. Am. Chem. Soc. 126, 3139–3147 (2004).
Fennimore, A. M. et al. Rotational actuators based on carbon nanotubes. Nature 424, 408–410 (2003).
Kassem, S., Lee, A. T. L., Leigh, D. A., Markevicius, A. & Solà, J. Pick-up, transport and release of a molecular cargo using a small-molecule robotic arm. Nat. Chem. 8, 138–143 (2016).
Jiménez, M. C., Dietrich-Buchecker, C. & Sauvage, J. Towards synthetic molecular muscles: contraction and stretching of a linear rotaxane dimer. Angew. Chem. Int. Ed. 39, 3284–3287 (2000).
Juluri, B. K. et al. A mechanical actuator driven electrochemically by artificial molecular muscles. ACS Nano 3, 291–300 (2009).
Kudernac, T. et al. Electrically driven directional motion of a four-wheeled molecule on a metal surface. Nature 479, 208–211 (2011).
Lehn, J. M. From supramolecular chemistry towards constitutional dynamic chemistry and adaptive chemistry. Chem. Soc. Rev. 36, 151–160 (2007).
Lehn, J. M. Perspectives in chemistry—steps towards complex matter. Angew. Chem. Int. Ed. 52, 2836–2850 (2013).
Ashton, P. R. et al. A light-fueled “piston cylinder” molecular-level machine. J. Am. Chem. Soc. 120, 11190–11191 (1998).
Saha, S. et al. A photoactive molecular triad as a nanoscale power supply for a supramolecular machine. Chem. Eur. J. 11, 6846–6858 (2005).
Lokey, R. S. & Iverson, B. L. Synthetic molecules that fold into a pleated secondary structure in solution. Nature 375, 303–305 (1995).
Wilson, H., Byrne, S. & Mullen, K. M. Dynamic covalent synthesis of donor-acceptor interlocked architectures in solution and at the solution:surface interface. Chem. Asian J. 10, 715–721 (2015).
Feldt, S. M. et al. Design of organic dyes and cobalt polypyridine redox mediators for high-efficiency dye-sensitized solar cells. J. Am. Chem. Soc. 132, 16714–16724 (2010).
Qin, P. et al. Design of an organic chromophore for p-type dye-sensitized solar cells. J. Am. Chem. Soc. 130, 8570–8571 (2008).
Olsen, J. C. et al. A neutral redox-switchable [2]rotaxane. Org. Biomol. Chem. 9, 7126–7133 (2011).
Fahrenbach, A. C. et al. Measurement of the ground-state distributions in bistable mechanically interlocked molecules using slow scan rate cyclic voltammetry. Proc. Natl Acad. Sci. USA 108, 20416–20421 (2011).
Nelson, J. J., Amick, T. J. & Elliott, C. M. Mass transport of polypyridyl cobalt complexes in dye-sensitized solar cells with mesoporous TiO2 photoanodes. J. Phys. Chem. C 112, 18255–18263 (2008).
Yella, A. et al. Dye-sensitized solar cells using cobalt electrolytes: the influence of porosity and pore size to achieve high-efficiency. J. Mater. Chem. C 5, 2833–2843 (2017).
Huang, Z., Natu, G., Ji, Z., Hasin, P. & Wu, Y. p-Type dye-sensitized NiO solar cells: a study by electrochemical impedance spectroscopy. J. Phys. Chem. C 115, 25109–25114 (2011).
Fabregat-Santiago, F., Bisquert, J., Palomares, E., Haque, S. A. & Durrant, J. R. Impedance spectroscopy study of dye-sensitized solar cells with undoped spiro-OMeTAD as hole conductor. J. Appl. Phys. 100, 034510 (2006).
Murakami, T. N. et al. Recombination inhibitive structure of organic dyes for cobalt complex redox electrolytes in dye-sensitised solar cells. J. Mater. Chem. A 1, 792–798 (2013).
Chang, Y.-C. et al. The influence of electron injection and charge recombination kinetics on the performance of porphyrin-sensitized solar cells: effects of the 4-tert-butylpyridine additive. Phys. Chem. Chem. Phys. 15, 4651–4655 (2013).
Luo, J., Wan, Z., Wang, Y. & Jia, C. A co-sensitization process for dye-sensitized solar cell: enhanced light-harvesting efficiency and reduced charge recombination. IOP Conf. Ser. Mater. Sci. Eng. 394, 042018 (2018).
D’Amario, L., Antila, L. J., Pettersson Rimgard, B., Boschloo, G. & Hammarström, L. Kinetic evidence of two pathways for charge recombination in NiO-based dye-sensitized solar cells. J. Phys. Chem. Lett. 6, 779–783 (2015).
Qin, P. et al. Synthesis and mechanistic studies of organic chromophores with different energy levels for p-type dye-sensitized solar cells. J. Phys. Chem. C 114, 4738–4748 (2010).
Zhu, K. et al. Unraveling the mechanisms of beneficial Cu-doping of NiO-based photocathodes. J. Phys. Chem. C 125, 16049–16058 (2021).
Atkins, P. & de Paula, J. Atkins’ Physical Chemistry (Oxford Univ. Press, 2010).
Pavlishchuk, V. V. & Addison, A. W. Conversion constants for redox potentials measured versus different reference electrodes in acetonitrile solutions at 25 °C. Inorg. Chim. Acta 298, 97–102 (2000).
Connelly, N. G. & Geiger, W. E. Chemical redox agents for organometallic chemistry. Chem. Rev. 96, 877–910 (1996).
Odobel, F. & Pellegrin, Y. Recent advances in the sensitization of wide-band-gap nanostructured p-type semiconductors. Photovoltaic and photocatalytic applications. J. Phys. Chem. Lett. 4, 2551–2564 (2013).
Acknowledgements
This study was supported by the Holland Research School for Molecular Sciences (HRSMC) and the University of Amsterdam. A part of this study was supported Merck GmbH and Dutch Research Council (NWO) for funding. The TA studies were supported by the Advanced Research Center for Chemical Building Blocks (ARC CBBC), which is cofounded and cofinanced by NWO and the Netherlands Ministry of Economic Affairs and Climate Policy. We thank AMOLF (FOM Institute for Atomic and Molecular Physics) for scanning electron microscopy imaging, W. Sikorski for the Brunauer–Emmett–Teller analysis of the NiO, M. Brands for her assistance with the TA measurements, E. von Hauff for her advice on the EIS measurements and S. Woutersen for his valuable contribution during discussions.
Author information
Authors and Affiliations
Contributions
T.B. and J.N.H.R. proposed the research. T.B. synthesized and characterized the dyes, the macrocycle and the pseudorotaxane with assistance from J.N.H.R. and M.D. The DSSC fabrication and characterization was performed by T.B. EIS measurements were performed by T.B. and analysed by T.M.A.B. TA experiments were designed by A.M.B., K.Z. and A.H. together with T.B., S.M. and J.N.H.R. TA measurements were performed by K.Z. and analysed by K.Z. and A.H. The remaining experiments were designed by T.B., S.M., T.M.A.B. and J.N.H.R. The manuscript was prepared by T.B., S.M. and J.N.H.R. with the assistance of T.M.A.B. and revised with the input of all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–37, Tables 1–16, Methods and experimental details.
Source data
Source Data Fig. 2
Unprocessed UV–Vis (binding) data.
Source Data Fig. 3
Unprocessed data on photovoltaic performance and EIS.
Source Data Fig. 4
Unprocessed femtosecond TA data, spectroelectrochemistry data and UV–Vis data.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bouwens, T., Bakker, T.M.A., Zhu, K. et al. Using supramolecular machinery to engineer directional charge propagation in photoelectrochemical devices. Nat. Chem. 15, 213–221 (2023). https://doi.org/10.1038/s41557-022-01068-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01068-y
This article is cited by
-
Embedding biocatalysts in a redox polymer enhances the performance of dye-sensitized photocathodes in bias-free photoelectrochemical water splitting
Nature Communications (2024)
-
Accelerating Oxygen Electrocatalysis Kinetics on Metal–Organic Frameworks via Bond Length Optimization
Nano-Micro Letters (2024)
-
Increased solar-driven chemical transformations through surface-induced benzoperylene aggregation in dye-sensitized photoanodes
Photochemical & Photobiological Sciences (2024)