ABSTRACT
We found T-type calcium channel blocker Ni2+ can efficiently induce the formation of cement gland in Xenopus laevis animal cap explants. Another T-type specific calcium channel blocker Amiloride can also induce the formation of cement gland, while L-type specific calcium channel blocker Nifedipine has no inductive effect. These results may offer us an new approach to study the differentiation of cement gland through the change of intracelluar calcium concentration.
Similar content being viewed by others
INTRODUCTION
The cement gland is a mucus-secreting organ located anterior most of the body axis of Xenopus laevis embryo. Although cement gland is itself a provisional and simple organ, research on the mechanism of its formation has important bearing in the understanding of anteroposterior pattern formation. The formation of cement gland can be easily influenced by dorsalizing, ventralizing, neuralizing or posteriorizing factors, either positively or negatively1, 2, 3, 4.
Although current research focused on the effect of secretory factors such as growth factors of TGF-β family, we tried to study this problem from a different way. As it has been reported that the signaling of the posteriorizing factor FGF is coupled with Ca2+ influx and without FGF signaling, the neuralizing factor Noggin induces the formation of cement gland rather than the expression of neural markers in animal cap explants5, we tried to use calcium channel blocker to study the possible effects of Ca2+ in this process. We have found that T-type calcium channel blocker Ni2+ can induce the formation of cement glands in animal cap explants. Another T-type specific calcium channel blocker Amiloride can also induce the formation of cement gland while L-type specific calcium channel blocker Nifedipine has no inductive effect. This phenomenon may offer us some insight into the intracellular mechanism involved in the differentiation of cement gland.
MATERIALS AND METHODS
The embryonic manipulation was carried out mainly according to Cold Spring Harbor Course Manual6. Briefly, Xenopus laevis female and male were both injected with 400 IU human chorionic gonadotrophin (hCG) and eggs were fertilized either naturally or in vitro. Embryos were dejellied in 2% cysteine (pH 7.8) and then transferred into Holtfreter solution. Embryos were incubated at room temperature(18-22°C )
The embryos were staged according to the time table of Nieuwkoop and Farber's7. The animal cap explants (animal pole ectoderm) were dissected from stage 9 embryos and cultured in 1× MBS (modified Barth's solution) with or without Ni2+ or other reagents. After different time of Ni2+ treatment, the animal cap explants were transferred into 1× MBS for further incubation.
NiCl2 and Amiloride were purchased from Sigma; Nifedipine was purchased from Shanghai Tian-ping Pharmaceutical Factory; other reagents were all of analytic grade.
RESULTS AND DISCUSSION
Ni 2+ can induce the formation of cement gland in animal cap explant
Animal cap explants from stage 9 embryos were routinely used for Ni2+ treatment in our experiment. From the data summarized in Fig 1, animal cap explants from stage 8-10 embryos could all be effciently induced to form cement gland by Ni2+ treatment, stage 9 embryos were preferred simply because they were easy to handle.
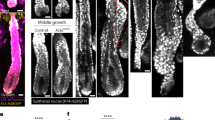
The optimal Ni2+ concentration was determined to be 100μM. From Tab 1, it can be seen that Ni2+ concentration from 20μM to 300μM can all induce the formation of cement gland, and the size of induced cement gland was proportional to the concentration of Ni2+ . The most strongly induced type was dubbed as "cigar" type, shown in Fig 2a. and Fig 3. At Ni2+concentration higher than 100μM, the outer layer had a tendency to separate form the inner layer. Treatment with extremely high concentration of Ni2+ could cause the dissociation of the animal cap.
Cement gland (arrow indicated) of different sizes, induced by different concentration of Ni2+.
a. the whole outer layer turned into cement gland, this type is dubbed as “cigar” type;
b. a patch of cement gland formed near the edge of the outerlayer, fre quently seen and so referred as nor mal sized cement gland;
c.a small sized spot of cement gland formed;
d. a "cigar" type type in its formation.
The induction of the formation of cement gland need at least 4 h of Ni2+ treatment after the animal cap was dissected from stage 9 embryo and directly treated with Ni2+ containing medium. It can be seen from Fig 4 that the effciency of induction did not change significantly from 4 h to overnight of Ni2+ treatment. But animal caps that were treated for only 3 h in Ni2+containing medium showed a drastically low effciency of cement gland induction.
However, when animal cap explants were first cultured in neutral saline such as MBS for 3 h and then transferred into Ni2+ ontaining medium for further incubation, the effciency of cement gland induction was no lower than those were directly cultured in Ni2+ containing medium after dissection. But animal cap explants being first cultured in MBS for 4 h instead of 3 h before they were transferred into Ni2+ containing medium, the result was entirely different. A drastic lowered effciency of induction happened. So it seems that the animal caps may have a time window for Ni2+ treatment between 3 and 4 h after being dissected from stage 9 embryos. We repeated this experiment for three times and results were in parallel.
The effect of calcium channel blockers on cement gland formation
Being a factor that has not often been used in biological research, especially in development biology, Ni2+ has little established biological effects. One of those known effects is that Ni2+ can specifically block the T-type calcium channel8, 9, 10, 11, 12, 13. The T-type calcium channel is unique for being a low-voltage gated calcium channel and its activation can cause prolonged rise of cytosolic calcium concentration. But whether this is causally related to the cement gland inducing ability of Ni2+ and whether there actually exists T-type calcium channel so early in the embryo are not known.
Since Nifedipine is a specific L-type calcium channel blocker12, and Amiloride blocks T-type calcium channel specifically, we used these two chemicals to further identify which kind of calcium channel is responsible in this phenomenon. We found even with concentration substantially higher than the Kd of Nifedipine (10 μM), there was no formation of cement gland at all. But Amiloride, another specific blocker of T-type calcium channel12, 14 could induce the formation of cement gland, although less effciently in comparison with Ni2+ treatment. About 50% of the animal cap explants treated with Amiloride formed cement glands of sizes that tended to be smaller than those found in Ni2+ treatment.
Possible mechanism of Ni 2+ treatment
Animal cap explants dissected from blastula embryos will form atypical epidermis if cultured in neutral medium such as MBS6, 15. The ability of Ni2+ to induce the animal cap explants to form cement gland may be resulted from the disturbance on certain induction or differentiation events of normal development. Ni2+ treatment may directly influence certain steps in the differentiation by changing the cytosol calcium concentration. Ni2+ wa reported to be a specific T-type calcium channel blocker and its Kd range from 30μM to 780μM in different systems8. We showed above that specific T-type calcium channel blocker Ni2+ and Amiloride can both induce the formation of cement gland while specific L-type calcium channel blocker Nifedipine has no effect on the development of cement gland. As the activation of T-type calcium channel was considered to result in sustained increase of cytosol calcium13, 16, the blockage of T-type calcium channel may disturb the calcium concentration fluctuation during normal development.
Therefore, a possible mechanism of Ni2+ treatment is that the blockage of the T-type calcium channel resulted in the hindrance of calcium influx which is vital for FGF signaling. The attenuated FGF signaling, in turn, cause the induction of cement gland. We are now trying to verify this possibility.
References
Sive H, Bradley L . A sticky problem: The Xenopus cement gland as a paradigm for anteroposterior patterning. Dev Dyn 1996; 205:265–80.
Bradley L, Wainstock D, Sive H . Positive and negative signals modulate formation of the Xenopus cement gland. Development 1996; 122:2739–50.
Sive H, Hattori K, Weintraub H . Progressive determination during formation of the anteroposterior axis in Xenopus laevis. Cell 1989; 58:171–80.
Gammill L, Sive H . Identification of otx2 target genes and restrictions in ectodermal competence during Xenopus cement gland formation. Development 1997; 124:471–81.
Amaya E, Musci T, Kirschner M . Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell 1991; 66:257–70.
Sive H, Grainger RM, Harland RM . In: Early Development of Xenopus laevis, Course Manual, Cold Spring Harbor fourth edition. 1996.
Nieuwkoop P, Faber J . In: Normal table of Xenopus laevis (Daudin), Amsterdam: North Holland. 1967.
Huguenard JR . Low-threshold calcium current in central nervous system neurons. Annu Rev Physiol 1996; 58:329–48.
Kaneda M, Akaike N . The low-threshold Ca current in isolated amygdaloid neurons in the rat. Brain Res 1989; 497:187–90.
Ye J, Akaike N . Calcium current in pyramidal neurons acutely dissociated from rat frontal cortex: a study by the nystatin perforated patch technique. Brain Res 1993; 606:111–7.
Mlinar B, Enyeart J . Block of current through T-type calcium channels by trivalent metal cations and nickel in neural rat and human cells. J Physiol 1993; 469:639–52.
De Waard M, Gurnett C, Campbell K . In: Narahashi T. eds. Ion Channel Volume 4. Plenum Press: New York 1996:41–87.
Tsien RW . Key clockwork component cloned. Nature 1998; 391:839–40.
Tang C, Presser F, Morad M . Amiloride selectively blocks the low threshold calcium channel. Science 1988; 240:213–5.
Sasai Y, De Robertis E . Ectodermal patterning in vertebrate embryos. Dev Biol 1997; 182:5–20.
Perez-Reyes E, Cribbs l, Paud A et al. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature 1998; 391:896–900.
Acknowledgements
We thank Lian Yin LIU, Qin Hua TAO and Wen Yan MEI for cooperation in the experiment, and Wei Gong HUANG for maintaining the animals.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
HUANG, Y., DING, X. Ni2+ treatment causes cement gland formation in ectoderm explants of Xenopus laevis embryo. Cell Res 9, 71–76 (1999). https://doi.org/10.1038/sj.cr.7290007
Issue Date:
DOI: https://doi.org/10.1038/sj.cr.7290007