Abstract
The amount and spatial organization of the heterochromatin in nuclei of the shoot meristem and the frequency in the nuclear DNA of sequences belonging to a family of tandem repeats were investigated in cultivars of Olea europaea and related species. Significant differences between Olea species and between cultivars of O. europaea were observed: (i) in the spatial organization of the heterochromatin in interphase nuclei as determined by the number and surface area of the chromocentres; (ii) in genome size; and (iii) in the amount of condensed chromatin as measured by cytophotometry carried out at different thresholds of optical density. DNA elements belonging to a family of tandem repeats about 80 bp in length (OeTaq80 repeats) were isolated from the genomic DNA of an olive cultivar. It was shown: (i) by nucleotide sequence comparisons, that these repeats display variability in structure even within the same array, where different elements may share no more than 74% homology; (ii) by in situ hybridization, that OeTaq80-related DNA sequences are mainly localized in the heterochromatin at the chromosome ends; (iii) by dot-blot hybridization experiments, that these sequences are highly represented in the genome of all the olive cultivars and the majority of Olea species studied, and that their frequency may differ significantly even between olive cultivars; and (iv) by calculating the copy number of OeTaq80-related sequences per haploid (1C) genome, that the redundancy of these DNA elements may differ significantly between the genomes tested. It is suggested that the inter- and intraspecific changes in the nuclear and genomic traits observed can contribute to the understanding of the phylogenetic relationships between Olea species and in defining parameters to be exploited in varietal identification within cultivated olives.
Similar content being viewed by others
Introduction
Olea europaea, the olive, is one of the oldest agricultural tree crops, and its cultural and economic importance is particularly remarkable in the countries of the Mediterranean Basin. In spite of this, to date, relatively little attention has been given to research on the cytology and molecular genetics of O. europaea. The chromosome number is known to be 2n=46 (Breviglieri & Battaglia, 1954) and the 1C DNA content of two cultivars, ‘Leccino’ and ‘Frantoio’, was estimated by Feulgen cytophotometry to be 2.26 pg and 2.20 pg, respectively (Rugini et al., 1996). Only very recently, after the results given in this paper were obtained, two tandemly repeated DNA sequences of Olea genomes have been characterized (Katsiotis et al., 1998). As a consequence of the shortage of information on the olive genome and its organization at the chromosomal level, there are conflicting hypotheses on the phylogenetic relationships between cultivated olives and related wild forms (Chevalier, 1948; Ciferri, 1950; Zohary, 1994), and our knowledge about the genetic diversity within O. europaea is only at a very initial stage (Pontikis et al., 1980; Loukas & Krimbas, 1983; Ouazzani et al., 1993; Fabbri et al., 1995; Trujillo et al., 1995). Furthermore, even though up to 2600 different olive cultivars have been recorded (Rugini & Lavee, 1992), varietal identification is still based on morphological traits, which may be misleading because of possible environmental effects on them.
In an attempt to reduce these knowledge gaps, we carried out a study of the heterochromatin in nuclei of Olea species and olive cultivars. A primary reason for studying heterochromatin is that its amount and spatial distribution is an important aspect of nuclear structure. It was suggested that, unlike in animals, the structural organization of cell nuclei in plants is function-independent and species-specific (Nagl, 1979). Therefore, nuclear changes may be indicative of given characteristics of the structure and organization of the genome and may help in evaluating intraspecific genetic diversity and phylogenetic relationships. A second reason is that the short sequences of highly repetitive DNA, which are mostly contained in the heterochromatin, are capable of relatively rapid changes in both structure and redundancy (Maggini et al., 1995). Therefore, comparisons of these sequences within and between species are informative about the evolution of genetic diversity within each of them and the creation of phylogenetic distances between them.
The heterochromatin of different Olea species and olive cultivars was examined by studying the nuclear structure with the aid of an image analyser, by differential staining of the chromatin, and by cytophotometry. Moreover, the DNA sequences belonging to a family of tandem repeats and their evolution in the genus Olea and within O. europaea were investigated.
Materials and methods
Plant material
Buds and leaves were collected from different Olea species and O. europaea cultivars and used for DNA extraction or fixed in ethanol–acetic acid 3 : 1 (v/v) to be used for cytological analyses. Root apices were collected from rooted cuttings of O. europaea cultivars treated with a saturated aqueous solution of alpha-bromonaphthalene for 4 h at room temperature, and fixed as above. At least three plants or rooted cuttings were sampled for each wild species or olive cultivar.
Light microscopy of cell nuclei
For observation under the light microscope, fixed materials were squashed under a coverslip in a drop of 45% acetic acid after treatment with a 5% aqueous solution of pectinase (Sigma) for 1 h at 37°C. The coverslips were removed by the solid CO2 method, and the preparations were Feulgen stained after hydrolysis in 1 N HCl at 60°C for 8 min or stained for 1 h with a 0.2% solution of DAPI (4,6-diamidino-2-phenylindole dihydrochloride) in McIlvaine buffer pH 7.0. Feulgen-stained preparations were dehydrated and mounted in DPX (BDH Chemicals). Those stained with DAPI were mounted in antifade solution (Citifluor). Nuclei and chromosomes were studied in images captured by a CCD camera using a Leica Q500MC.
DNA cytophotometry
Fixed buds were squashed and Feulgen stained as described above. After staining, the slides were subjected to three 10-min washes in SO2 water prior to dehydration and mounting in DPX. Feulgen/DNA absorptions in individual cell nuclei were measured, at the wavelength of 550 nm, by a Leitz MPV3 microscope photometer equipped with a mirror scanner after selecting different thresholds of optical density. The instrument does not read any part of the nucleus where the optical density is lower than the preselected limit. Squashes made with the root tips of Sorghum bicolor (1C=0.8 pg; Bennett & Smith, 1991) were stained concurrently with the other preparations and used as standards in order to make the results comparable and to convert the relative Feulgen units into picograms of DNA.
DNA extraction, fractionation and cloning
Nuclear DNA was extracted and purified according to Doyle & Doyle (1991). Genomic DNA was digested to completion with AluI restriction endonuclease (Boehringer) and fractionated by electrophoresis in a 1% agarose gel. The DNA in a band of about 21 kb, which was visualized under UV light after ethidium bromide staining, was recovered from a preparative 2% agarose gel and digested with TaqI (Boehringer).
The digested DNA was ligated in the presence of pBS/AccI BAP, and the ligation mixture was used to transform Escherichia coli DH5alpha.
DNA sequencing and probe preparation
The insert of one recombinant clone was sequenced using the dideoxy method of Sanger et al. (1977) as modified by Chen & Seburg (1985) for double-stranded plasmid DNA.
A DNA fragment of seven tandem repeats, each about 80 bp in length, was subcloned in pBS and used as a probe in molecular and cytological hybridization experiments.
In situ hybridization
Alpha-bromonaphthalene-treated root apices were squashed as described above after treatment with a solution of pectinase (20%; Sigma) and cellulase (4%; Calbiochem) in citrate buffer pH 4.6 for 2 h at 37°C. The preparations were then processed and in situ hybridization was performed according to Schwarzacher et al. (1988). The DNA of nuclei and chromosomes was denatured in 70% (v/v) deionized formamide in 2 × SSC for 2 min at 70°C, and the preparations were incubated overnight with 2 ng/μL of heat-denatured DNA probe, which was labelled with digoxigenin–dUTP by random priming using a commercial kit (Boehringer). The digoxigenin at the hybridization sites was detected by using sheep antidigoxigenin fluorescein (Boehringer). The preparations were then counterstained with DAPI and mounted as described above.
Dot-blot hybridization and calculation of sequence copy number
Replicated samples of 15, 30 or 60 ng of genomic DNA were suspended in 10 μL of TE buffer (0.01 M Tris–HCl, pH 8.0, plus 0.001 M EDTA, pH 8.0) and applied to Zeta-Probe (Bio-Rad) filters using a Minifold I apparatus (Schleicher & Schuell). To the filters, 5 × 108, 5 × 109 or 5 × 1010 of probe DNA sequences were also applied (DNA probe dilution spots). The filters were processed and scanned densitometrically after hybridization as described previously (Ceccarelli et al., 1995). Hybridization was performed under two stringency conditions (60°C or 65°C; 5 × SSC in the hybridization mixture) using 50 ng per filter of DNA probe that had been labelled with digoxigenin as described above. Digoxigenin haptens in DNA–DNA hybrids were detected using a Dig-DNA detection kit (Boehringer) by enzyme-linked immunoassay using an antibody conjugate (antidigoxigenin–alkaline phosphate conjugate).
The linear regression equation, relating the natural logarithm of the copy number of probe sequences in the dilution spots and the natural logarithm of the corresponding densitometric readings, was used to calibrate the relationship between the copy number of the sequence probed in the samples of genomic DNA and the amount of absorbance as detected densitometrically.
Results
Amount and organization of the heterochromatin in nuclei of the shoot meristem
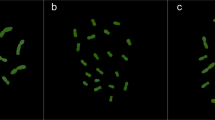
The chromocentric structure of cell nuclei is common to all the Olea species studied. Prominent chromatin structures (chromocentres) can be seen in interphase nuclei after Feulgen staining (Fig. 1a), and more numerous heterochromatic nuclear regions appear after DAPI staining, because even very small chromocentres can be detected by the fluorochrome (Fig. 1b–e). By observing DAPI-stained metaphase chromosomes (Fig. 1f) it appears that all chromosomes show at least one positive band and that the heterochromatin is located mainly at the telomeres. Only two chromosome pairs do not show DAPI-positive bands at their ends, and five pairs have pericentromeric or interstitial DAPI bands in addition to telomeric ones. It can be seen from Fig. 1 that the number per nucleus and the surface area of the chromocentres may differ between Olea species or olive cultivars. Extensive analysis of these two nuclear parameters was carried out in five of the latter and five wild Olea entities. The number and surface area of heterochromatic nuclear portions were recorded in interphase nuclei of the bud meristem on the basis of their optical density by using the image analyser. Nuclei of comparable size which were close to mitoses in the preparations were chosen. Fig. 2 shows that a highly significant, negative correlation exists between the mean number of chromocentres per nucleus and their mean surface area in each species or cultivar. Therefore, the fewer the chromocentres, the larger they are. This suggests that the heterochromatic regions of different chromosomes may associate closely, and our data show that this aspect of the spatial organization of the heterochromatin may undergo species- or cultivar-specific variations. Indeed, the number and size of the chromocentres may differ significantly between species or cultivars (see confidence limits in Fig. 2). It also appears from Fig. 2 that chromocentre association occurs to a lesser extent in all the olive cultivars studied than in the wild species. It may also be worth noting that O. europaea var. sylvestris lies between the two groups of entities as far as the nuclear character examined is concerned.
Correlation, in five Olea species and five olive cultivars, between the mean number of chromocentres and their mean surface area in Feulgen-stained nuclei of the shoot meristem. Each point is the mean of the values obtained by studying 50 nuclei. 1, cv. ‘Moresca’; 2, cv. ‘Dolce Agogia’; 3, cv. ‘Frantoio’; 4, cv. ‘Pendolino’; 5, cv. ‘Leccino’; 6, O. europaea var. sylvestris; 7, O. indica; 8, O. africana; 9, O. ferruginea; 10, O. cuspidata. Bars represent 99% confidence limits.
Feulgen cytophotometric determinations carried out at different thresholds of optical density showed that the percentage of dense chromatin in interphase nuclei may differ remarkably between Olea species or olive cultivars (Fig. 3a,b). Therefore, not only the spatial organization of the heterochromatin, but also its relative amount may constitute a distinctive nuclear character that differentiates Olea entities.
Characterization of a family of tandem repeated DNA sequences
When electrophoresed after digestion with AluI, the DNA of O. europaea cv. ‘Carolea’ produced a high molecular weight band of about 21 kb (not shown). A clone obtained from the DNA of this band after cleaving with TaqI was a tandem array of seven repeats about 80 bp in length (hereinafter termed OeTaq80 repeats), which produced the band pattern shown in Fig. 4 when hybridized to Southern blots of TaqI-digested genomic DNA of the same cultivar. The nucleotide sequences of the repeats in this array are given in Fig. 5. It appears that they vary in length and structure. The length fluctuated from 76 to 80 bp; the homology between the two most similar DNA elements was about 91% and that between the two most differing elements was about 74%. About 80% nucleotide sequence homology occurs between OeTaq80 repeats and O. europaea DNA elements of similar length recently described by Katsiotis et al. (1998). No significant homology was found after comparison with other DNA sequences in the PC/Gene program or in the EMBL/GenBank/DDBJ nucleotide sequence databases.
The results obtained by hybridizing OeTaq80 repeats to metaphase chromosomes of O. europaea cv. ‘Leccino’ are shown in Fig. 6(b). It appears that these DNA sequences are localized mostly at the chromosome ends. Out of the 23 chromosome pairs, 11 bear OeTaq80 repeats at one telomere, and seven pairs at both telomeres. Only three chromosome pairs bear OeTaq80 repeats at nontelomeric regions, and two pairs do not show any hybridization signal. By observing Fig. 6c, where DAPI banding and the chromosomal localization of OeTaq80-related DNA sequences are overlapped, it appears that the latter overlap DAPI bands (reddish). However, nontelomeric, DAPI-positive chromosome regions do not show any detectable hybridization signal (blue), except in two chromosome pairs. One of them is devoid of telomeric DAPI bands, and the other bears OeTaq80 repeats at an interstitial region in the longer arm in addition to a DAPI band at the end of the shorter arm.
Metaphase of Olea europaea cv. ‘Leccino’ after (a) DAPI staining or (b) hybridization with OeTaq80 DNA repeats (FITC staining). The two images are electronically overlaid in (c), where DAPI-positive chromosome regions that bear OeTaq80-related DNA sequences are reddish, whereas those devoid of OeTaq80 repeats are blue. ×1500.
The results of dot-blot hybridizations of OeTaq80-repeats to the genomic DNA of Olea species and olive cultivars are given in Fig. 7. It can be seen that these DNA sequences were highly represented in all the latter genomes and in most of the former, and that their frequency may differ significantly even between the olive cultivars studied. The genome size of four Olea species and three olive cultivars was calculated from the results of Feulgen cytophotometry. Fig. 8 shows the copy number of DNA sequences related to OeTaq80 repeats to differ significantly between these genomes, and to be positively correlated with the genome size.
Frequencies of sequences related to OeTaq80 repeats in the DNAs of different Olea species and olive cultivars as calculated from the results of dot-blot hybridizations carried out under higher (▮) or lower (▨) stringency conditions. Each value is the mean of six replicates, three for each of two DNA extractions. Bars represent 99% confidence limits. 1, O. cuspidata; 2, O. ferruginea; 3, O. chrysophylla; 4, O. africana; 5, O. indica; 6, O. maroccana; 7, O. laperrinei; 8, O. europaea var. sylvestris; 9, O. lancea; 10, O. paniculata; 11, ‘Sigoise’; 12, ‘Chetoui’; 13, ‘Arbequina’; 14, ‘Ayvalik’; 15, ‘Leccino’; 16, ‘Pendolino’; 17, ‘Dolce Agogia’; 18, ‘Tonda Iblea’; 19, ‘Frantoio’; 20, ‘Moresca’; 21, ‘Koroneiki’; 22, ‘Valanolia’; 23, ‘Carolea’.
Correlation between the copy number of OeTaq80-related DNA sequences (Fig. 7; higher stringency) and the DNA content in the haploid (1C) genome of Olea species and olive cultivars. Feulgen cytophotometry was carried out, in each accession, on 30 early prophases in the root meristem. 1, O. africana; 2, O. indica; 3, O. ferruginea; 4, cv. ‘Dolce Agogia’; 5, O. cuspidata; 6, cv. ‘Frantoio’; 7, cv. ‘Pendolino’. Bars represent 99% confidence limits.
Duncan grouping at P=0.01 of Olea species on the basis of the frequency of OeTaq80-related DNA sequences in their genomes (as calculated from the results of molecular hybridizations carried out under high stringency conditions) allowed us to separate the wild forms studied into three groups. The first group comprised accessions from Asia and Africa, namely O. africana, O. chrysophylla, O. cuspidata, O. ferruginea and O. indica (from 21.60 × 108 to 25.00 × 108 copies per ng of DNA); the second group was made up of accessions from the Saharan region, namely O. laperrinei and O. maroccana and the only O. europaea var. sylvestris studied, which comes from Balearic Islands (from 12.50 × 108 to 14.70 × 108 copies); and the third group included O. lancea from Mauritius and O. paniculata from Australia (2.30 × 108 and 0.75 × 108 copies, respectively).
On the same basis, olive cultivars could be split into two groups. The smaller one comprised cv. ‘Carolea’, ‘Koroneiki’ and ‘Valanolia’, which are cultivated in Greece and south-east Italy. In these genomes, the frequency of OeTaq80-related DNA sequences ranged from 15.15 × 108 to 20.50 × 108 copies per ng of DNA. The larger group included all the remaining cultivars studied. In their genomes, the frequency of OeTaq80-related sequences ranged from 30.50 × 108 to 43.40 × 108 copies per ng of DNA.
Discussion
Traits of nuclear structure and genome organization may differ between wild Olea forms and between the cultivars of O. europaea. Indeed, the results of our cytological investigation showed that differences may occur in certain characteristics of the heterochromatin such as its amount (Fig. 3), and spatial organization in interphase nuclei (Figs. 1 and Fig. 2). Moreover, biochemical analyses indicated significant differences in the frequency in the nuclear DNA of tandem repeats which were localized in the heterochromatin and which had homology to a probe taken from the genomic DNA of O. europaea cv. ‘Carolea’ (Fig. 7). Data from cytological and molecular analyses are in agreement, corroborating the above statements. Indeed, the species in which the amount of heterochromatin was relatively high also had a relatively high frequency of OeTaq80-related DNA sequences in their genome: e.g. O. cuspidata (Figs. 3 and Fig. 7). DNA contents as determined by cytophotometry were positively correlated with the copy number of DNA repeats in the genomes (Fig. 8).
The above findings indicated that the genomes studied may differ in their amounts of repetitive DNA. Changes in the redundancy of repeated sequences in the nuclear DNA have been shown to play a part in speciation (e.g. Narayan & Rees, 1976). Moreover, these genomic alterations have recently been proved to differentiate cultivars, natural populations, individuals or even organs of the same individual in other plant species, such as Helianthus annuus (Cavallini et al., 1986, 1996), Festuca arundinacea (Ceccarelli et al., 1992), Vicia faba (Ceccarelli et al., 1995; Maggini et al., 1995), Dasypyrum villosum (Frediani et al., 1994; Caceres et al., 1998), or Trapa natans (Bitonti et al., 1996).
The alterations observed in the nuclear traits studied may help us to understand the relationships between species within the Olea genus and the phylogenesis of the cultivated olive. Regarding the first problem, our grouping of wild Olea forms on the basis of the frequency in their genomes of OeTaq80-related DNA sequences agrees well with the systematics of the O. europaea complex as proposed by Green & Wickens (1988). Indeed, these authors consider the Asiatic and African forms we studied to be ranked together under O. europaea ssp. cuspidata, and rank the Saharan forms under a different subspecies of O. europaea, laperrinei. On the same basis, the olive cultivars studied can also be divided into two groups. Taking into account that the frequency of OeTaq80-related DNA sequences is generally higher in the cultivated varieties than in the wild forms (Fig. 7), each of the two groups of cultivars seems to share genomic similarity with two different groups of wild forms. This suggests that different cultivars may be derived from, or have introgressed DNA elements of, different wild Olea entities.
Our results showed that nuclear traits such as the DNA content (Fig. 8) and the spatial organization (Fig. 2) or the amount (Fig. 3) of the heterochromatin may differ significantly between olive cultivars. Possibly, differences in the banding pattern of metaphase chromosomes and the chromosomal distribution of DNA repeats will be found after comparing cultivars in this respect. Therefore, these nuclear traits, together with other characters of the genome such as the redundancy levels of given repeated DNA sequences may provide suitable parameters for varietal identification within cultivated olives. Cytological analyses are less expensive, as far as both money and time are concerned, and need less complex and sophisticated equipment than analyses of genomic traits that use isozyme or DNA markers. These advantages may be of particular interest in certain countries where olives are cultivated.
References
Bennett, M. D. and Smith, J. B. (1991). Nuclear DNA amounts in angiosperms. Phil Trans R Soc B, 334: 309–345.
Bitonti, M. B., Cozza, R., Wang, G., Ruffini-castiglione, M., Mazzuca, S., Castiglione, S. et al (1996). Nuclear and genomic changes in floating and submerged buds and leaves of heterophyllous waterchestnut (Trapa natans). Physiol Plant, 97: 21–27.
Breviglieri, N. and Battaglia, E. (1954). Ricerche cariologiche in O. europaea L. Caryologia, 61: 271–283.
Caceres, M. E., Depace, C., Scarasciamugnozza, G. T., Kotsonis, P., Ceccarelli, M. and Cionini, P. G. (1998). Genome size variations within Dasypyrum villosum: correlations with chromosomal traits, environmental factors and plant phenotypic characteristics and behaviour in reproduction. Theor Appl Genet, 96: 559–567.
Cavallini, A., Zolfino, C., Cionini, G., Cremonini, R., Natali, L. and Sassoli, O. et al (1986). Nuclear DNA changes within Helianthus annuus L.: cytophotometric, karyological and biochemical analyses. Theor Appl Genet, 73: 20–26.
Cavallini, A., Natali, L., Giordani, T., Durante, M. and Cionini, P. G. (1996). Nuclear DNA changes within Helianthus annuus L.: variations in the amount and methylation of repetitive DNA within homozygous progenies. Theor Appl Genet, 92: 285–291.
Ceccarelli, M., Falistocco, E. and Cionini, P. G. (1992). Variation of genome size and organization within hexaploid Festuca arundinacea. Theor Appl Genet, 83: 273–278.
Ceccarelli, M., Minelli, S., Maggini, F. and Cionini, P. G. (1995). Genome size variation within Vicia faba. Heredity, 74: 180–187.
Chen, E. Y. and Seburg, P. H. (1985). Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA (New York), 4: 167–170.
Chevalier, A. (1948). L’origine de l’olivier cultivée et ses variations. Rev Int Bot Appl Agric Trop, 28: 1–25.
Ciferri, R. (1950). Dati ed ipotesi sull’origine e l’evoluzione dell’olivo. Olearia, 4: 115–122.
Doyle, J. J. and Doyle, J. L. (1991). Isolation of plant DNA from fresh tissue. Focus, 12: 13–15.
Fabbri, A., Hormaza, J. I. and Polito, V. S. (1995). Random amplified polymorphic DNA analysis of olive (Olea europaea L.) cultivars. J Am Soc Hort Sci, 120: 538–542.
Frediani, M., Colonna, N., Depace, C., Delre, V., Caccia, R. and Cionini, P. G. (1994). Redundancy modulation of nuclear DNA sequences in Dasypyrum villosum. Theor Appl Genet, 88: 167–174.
Green, P. S. and Wickens, G. I. (1989). The Olea europaea complex. In: Kit Tan (ed.) The Davis and Hedge Festschrift pp. 287–299. Edinburgh University Press, Edinburgh.
Katsiotis, A., Hagidimitriou, M., Douka, A. and Hatzopoulos, P. (1998). Genomic organization, sequence interrelationship, and physical localization using in situ hybridization of two tandemly repeated DNA sequences in the genus Olea. Genome, 41: 527–534.
Loukas, M. and Krimbas, C. B. (1983). History of olive cultivars based on their genetic distances. J Hort Sci, 58: 121–127.
Maggini, F., D’ovidio, R., Gelati, M. T., Frediani, M., Cremonini, R. and Ceccarelli, M. et al (1995). FokI DNA repeats in the genome of Vicia faba: species specificity, structure, redundancy modulation, and nuclear organization. Genome, 38: 1255–1261.
Nagl, W. (1979). Condensed interphase chromatin in plant and animal cell nuclei: fundamental differences. Pl Syst Evol. (Suppl.) 2: 247–260.
Narayan, R. K. J. and Rees, H. (1976). Nuclear DNA variation in Lathyrus. Chromosoma, 54: 141–154.
Ouazzani, N., Lumaret, R., Villemur, P. and Digiusto, F. (1993). Leaf allozyme variation in cultivated and wild olive trees (Olea europaea L.). J Hered, 84: 34–42.
Pontikis, C. A., Loukas, M. and Koussounis, G. (1980). The use of biochemical markers to distinguish olive cultivars. J Hort Sci, 55: 333–343.
Rugini, E. and Lavee, S. (1992). Olive. In: Hammerschlag, F. A. and Litz, R. E. (eds) Biotechnology of Perennial Fruit Crops. pp. 371–382. C.A.B. International, Wallingford, U.K..
Rugini, E., Pannelli, G., Ceccarelli, M. and Muganu, M. (1996). Isolation of triploid and tetraploid olive (Olea europaea L.) plants from mixoploid cv. ‘Frantoio’ and ‘Leccino’ mutants by in vivo and in vitro selection. Pl Breed, 115: 23–27.
Sanger, F., Nicklen, S. and Coulson, A. R. (1977). DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA, 74: 5463–5467.
Schwarzacher, T., Leitch, A. R., Bennett, M. D. and Heslop-Harrison, J. S. (1989). In situ localization of parental genomes in a wide hybrid. Ann Bot, 64: 315–324.
Trujillo, I., Rallo, L. and Arus, P. (1995). Identifying olive cultivars by isozyme analysis. J Am Soc Hort Sci, 120: 318–324.
Zohary, D. (1994). The wild genetic resources of the cultivated olive. Acta Hort, 356: 62–65.
Acknowledgements
Research was supported by UE-MURST/CNR, project OEVOCAL and the EC FAIR programme, contract no. CT95–0689
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bitonti, M., Cozza, R., Chiappetta, A. et al. Amount and organization of the heterochromatin in Olea europaea and related species. Heredity 83, 188–195 (1999). https://doi.org/10.1046/j.1365-2540.1999.00564.x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1046/j.1365-2540.1999.00564.x
Keywords
This article is cited by
-
Identification and characterisation of Short Interspersed Nuclear Elements in the olive tree (Olea europaea L.) genome
Molecular Genetics and Genomics (2017)
-
Genome sequence of the olive tree, Olea europaea
GigaScience (2016)
-
Molecular–cytogenetic studies of ribosomal RNA genes and heterochromatin in three European Fraxinus species
Tree Genetics & Genomes (2014)
-
Molecular studies in olive (Olea europaea L.): overview on DNA markers applications and recent advances in genome analysis
Plant Cell Reports (2011)
-
Nuclear DNA content estimations in wild olive (Olea europaea L. ssp. europaea var. sylvestris Brot.) and Portuguese cultivars of O. europaea using flow cytometry
Genetic Resources and Crop Evolution (2007)