Abstract
LTR-retrotransposons, knobs and structural chromosome alterations contribute to shape the structure and organization of the Zea mays karyotype. Our initial nuclear DNA content data of Z. mays accessions revealed an intraspecific variation (2 C = 2.00 pg to 2 C = 6.10 pg), suggesting differences in their karyotypes. We aimed to compare the karyotypes of three Z. mays accessions in search of the differences and similarities among them. Karyotype divergences were demonstrated among the accessions, despite their common chromosome number (2n = 20) and ancestral origin. Cytogenomic analyses showed that repetitive sequences and structural chromosome alterations play a significant role in promoting intraspecific nuclear DNA content variation. In addition, heterozygous terminal deletion in chromosome 3 was pointed out as a cause of lower nuclear 2 C value. Besides this, translocation was also observed in the short arm of chromosome 1. Differently, higher 2 C value was associated with the more abundant distribution of LTR-retrotransposons from the family Grande in the karyotype. Moreover, heteromorphism involving the number and position of the 180-bp knob sequence was found among the accessions. Taken together, we provide insights on the pivotal role played by repetitive sequences and structural chromosome alterations in shaping the karyotype of Z. mays.
Similar content being viewed by others
Introduction
The genus Zea is a group of annual and perennial grasses native to a region extending from Mexico to Central America1. Based on morphological traits, geographical distribution and genomic data, five Zea species are currently recognized: the closely related perennial species Zea diploperennis Iltis, Doebley & Guzman (2n = 2×= 20) and Zea perennis (Hitchcock) Reeves & Mangelsdorf (2n = 4×= 40); the annual species Zea luxurians (Durieu & Ascherson) Bird; Zea nicaraguensis Iltis & Benz; and Zea mays L. (2n = 2×= 20). Z. mays is divided into four subspecies: (i) ssp. parviglumis Iltis & Doebley, which represents the direct progenitor of Z. mays ssp. mays1,2,3; (ii) ssp. mays, a well-studied cereal crop with extensive genetic diversity, commonly known as maize or corn4; (iii) ssp. huehuetenangensis (Iltis & Doebley) Doebley; and (iv) ssp. mexicana (Schrader) Iltis. Although controversies still exist regarding the origin of maize, allozyme genetic analyses5,6 and simple sequence repeat (SSR) markers7 have provided strong evidence to support Z. mays ssp. parviglumis as the progenitor of Z. mays ssp. mays.
Transposable elements (TEs) contribute to the dynamics of the nuclear genome, either through polymorphic insertions and deletions or by mediating ectopic recombination events that can drive structural variation in the genome8. TEs constitute over 85% of the maize reference (B73) genome4; of these, ~70% belong to Class-I long terminal repeat (LTR) retrotransposons, which replicate through an RNA intermediate, as in the superfamilies Gypsy and Copia8. The families Huck, Cinful, Tekay/Prem-1 and Grande belong to the superfamily Gypsy, while Prem-2/Ji and Opie are included in Copia8,9. These families constitute a large fraction of the Z. mays genome and are distributed throughout its ten chromosomes10, but predominantly mapped in heterochromatic regions9. The cytogenetic determination of the genomic distribution of retrotransposons achieved to date in Z. mays9,10 has evinced their abundance and physical location in the chromosomes.
The genomic dynamism of the LTR-retrotransposon families, which has been characterized by amplification and loss, is responsible for the variation in genome size among Z. mays accessions11,12 that ranges from 2 C = 4.50 to 7.11 pg13,14. DNA content divergences have also been shown at chromosome level employing image cytometry. The DNA content has been measured for the long and short arms of each chromosome and the satellite of chromosome 6 of Z. mays ‘AL Bandeirante’15. The DNA content of chromosome 9 (2 C = 0.56 pg) was higher than that of chromosome 8 (2 C = 0.53 pg)15, a fact that can be related to accumulation of repetitive sequences in the knobs16. Hence, intraspecific variation in nuclear or chromosomal DNA content hints at karyotype differences, emphasizing the need to understand the causes of these variations in distinct Z. mays accessions.
In addition to TEs, knobs are also responsible for the intraspecific variation in Z. mays nuclear genome size17,18. Knobs are heterochromatic regions identified in pachytene and mitotic prometaphase and metaphase chromosomes by means of differential staining techniques19. They are composed of two tandem repeat sequences, of 180 base pairs (bp)20 and 350 bp (TR-1), besides harboring several LTR–retrotransposons17,21. The positions, number and size of the knobs are variable among both the accessions and the chromosomes of the same karyotype9,22. In some cases, the knobs might serve as chromosomal markers that provide physical evidence of crossing-over events between non-homologous chromosomes23. Despite the recognized role of TEs and knobs on the dynamism of Z. mays genome size variation, little is known about the important of these sequences on plant fitness. However, Bilinski et al.18 have found evidences that this variation may indeed be adaptive and that heterochromatic knob sequences are likely under the effect of natural selection. Therefore, considering that knob heteromorphism correlates with nuclear genome size, it is fundamental to map these portions in Z. mays chromosomes in order to verify their involvement in DNA content divergence.
In addition to LTR–retrotransposons and knobs, the chromosome structure and morphology can also be altered by structural rearrangements: duplication, deletion, translocations and/or inversions24. These rearrangements have been revealed in the genera Solanum25, Brachypodium26 and Z. mays27 by comparative cytogenetics via chromosome painting, thus assisting the elucidation of their evolutionary histories. During the process of double-strand break repair, several rearrangements may occur as a result of illegitimate recombination or through recombination of homologous ectopic sequences28. Thus, repetitive sequences such as TEs can provide a template for repairing the double-strand breaks. For this reason, heterochromatic regions rich in similar repetitive sequences are considered hotspots for double-strand breaks29,30.
Beyond the karyotype diversity and dynamism of Z. mays ssp. mays, this taxon also occupies a wide range of habitats and presents a diversity of morphological traits1,31, being a crop with several agricultural varieties specific for different uses31. For example, the popcorn is characterized by small, hard kernels that explode when heated, forming large flakes (popping expansion), the major feature that separates popcorn from other types of maize31,32. Sturtevant33 considered popcorn as a distinct species, Zea everta, which was posteriorly reduced to a subspecies34 and then considered as a mutant of flint maize35. However, archeological evidence and the quantitative trait of popping ability rendered improbable the hypothesis of a mutant origin from flint maize32. Currently, taxonomists consider that popcorn belongs to the taxon Z. mays ssp. mays (https://www.itis.gov/about_itis.html; http://www.plantsoftheworldonline.org). The origin and evolutionary relationship of popcorn with other types of maize remains unknown31. Therefore, the genomic in situ hybridization (GISH) and the comparative chromosome painting via chromosome-specific probes might to discriminate the homologous chromosome regions, contributing to understand the evolutionary relationships of popcorn.
Considering the remarkable karyotype dynamism, intraspecific variation in nuclear genome size and chromosomal DNA15,36 within Z. mays, the aim of this study was to perform a comparative analysis of the karyotypes of different Zea accessions, seeking to identify if the nuclear genome size variation among them is promoted by differential amounts of repetitive sequences and/or by structural chromosomal rearrangements.
Results
G0/G1 peaks of Z. diploperennis and ‘Milho Pipoca Americano RS 20’ overlapped with the internal standard Z. mays ‘CE-777’, even with coefficients of variation between 2.91% and 4.77%. Because these overlapped G0/G1 peaks compromise the reliability, we also utilized the internal standard S. lycopersicum, thus avoiding this flow cytometry problem. Z. diploperennis showed 2 C = 5.76 ± 0.06 pg, corresponding to 2 C = 5.63 × 109 bp, whereas ‘Milho Pipoca Americano RS 20’ showed mean 2 C = 5.55 ± 0.6 pg, corresponding to 2 C = 5.43 × 109 bp. For the popcorn ‘15-1149-1’ and ‘AL Bandeirante’, the nuclear 2 C value was measured with the internal standard ‘CE-777’ and with S. lycopersicum. Popcorn ‘15-1149-1’ presented 2 C = 2.00 ± 0.17 pg, corresponding to 2 C = 1.96 × 109 bp, and ‘AL Bandeirante’ showed 2 C = 6.10 pg, corresponding to 2 C = 5.97 × 109 bp (Supplementary Fig. 1). Thus, the mean 2 C value of Z. diploperennis was 2 C = 3.76 pg higher than ‘15-1149-1’, 2 C = 0.21 pg higher than ‘Milho Pipoca Americano RS 20’, and 2 C = 0.34 pg lower than ‘AL Bandeirante’. ‘Milho Pipoca Americano RS 20’ presented 2 C = 3.55 pg higher than ‘15-1149-1’, and 2 C = 0.55 pg lower than ‘AL Bandeirante’. In a similar trend, ‘15-1149-1’ exhibited 2 C = 4.10 pg lower than ‘AL Bandeirante’.
Given these nuclear genome size differences, we explored the karyotypes in order to understand the causes of these divergences among Zea accessions. For this, a metaphasic index of 60% was obtained from the cell cycle arrest, achieved by treatment involving hydroxyurea followed by amiprophos-methyl. Besides, to ensure morphologically preserved chromosomes with well-defined telomeres and primary constrictions, enzymatic maceration of root meristems and air-drying technique were used for slide preparation.
Once the differences in nuclear genome size were verified, the genomic homology among Z. mays accessions was confirmed. Even with GISH stringency at 85–90%, all chromosomes of at least ten ‘Milho Pipoca Americano RS 20’ and ‘AL Bandeirante’ metaphases were fully hybridized by the genomic probe of Z. diploperennis. The same result was observed for the genomic probe of ‘Milho Pipoca Americano RS 20’ applied to the ‘AL Bandeirante’ karyotype (Fig. 1). From hybridization of our previously constructed probe of Z. mays ‘AL Bandeirante’ chromosome 1, the homology between Z. mays accessions was also evidenced by specific painting of the chromosome 1 of ‘Milho Pipoca Americano RS 20’ (Supplementary Fig. 2). Therefore, these results provide substantial evidence that popcorn belongs to the subspecies mays.
GISH in metaphase chromosomes of Z. mays labelled with ChromaTide-488-5-dUTP (green). (a) ‘AL Bandeirante’ chromosomes fully labelled by the genomic probe from ‘Milho Pipoca Americano RS 20’. (b) ‘AL Bandeirante’ and (c) ‘Milho Pipoca Americano RS 20’ chromosomes fully labeled by the genomic probe of Z. diploperennis. Bar = 10 µm. Images were digitized using the Image-Pro Plus software version 6.1 (https://www.mediacy.com/imageproplus).
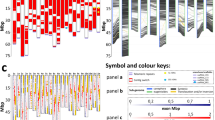
Nevertheless, karyotype differences were demonstrated among the Z. mays ssp. mays accessions. Structural chromosome changes were identified in all karyotypes of ‘15-1149-1’, which were specifically stained by Feulgen reaction, DAPI and labeled by the 180-bp probe. Two alterations were identified (Supplementary Fig. 3): terminal deletion in the long arm of chromosome 3 and translocation in the short arm of chromosome 1 (Fig. 2a). The translocation was identified as one detectable chromosome fragment, but it was not classified as non-reciprocal or reciprocal. Differently, no structural chromosomal aberration was observed in ‘Milho Pipoca Americano RS 20’ and ‘AL Bandeirante’ karyotypes (Fig. 2b,c).
Differential DAPI staining (blue) in two metaphases of (a) ‘15-1149-1’, (b) ‘Milho Pipoca Americano RS 20’ and (c) ‘AL Bandeirante’. (a) Structural chromosome alterations in ‘15-1149-1’: translocation in the short arm of chromosome 1 and heterozygous terminal deletion in the long arm of chromosome 3. (b,c) In ‘Milho Pipoca Americano RS 20’ and ‘AL Bandeirante’, no structural chromosomal aberration was observed. The DAPI-banding pattern in Z. mays chromosomes was promoted by the preferential binding of this fluorochrome to A-T rich sequences, allowing to evidence the knob portions in a cyan blue color. Bar = 10 µm. Images were digitized using the Image-Pro Plus software version 6.1 (https://www.mediacy.com/imageproplus).
Apart from these structural chromosome aberrations, karyotype variations were also found regarding the number and position of the 180-bp knob sequence, including heteromorphism within the same karyotype between the chromosome pair (Fig. 3). The 180-bp sequence was mapped in nine different chromosome portions (1 L, 2 L, 3 L, 4 S, 4 L, 5 S, 5 L, 6 L or 8 L) in the karyotypes of Z. mays ‘15-1149-1’ and ‘AL Bandeirante’. Z. mays ‘15-1149-1’ exhibited positive signals in the chromosome portions 3 L, 4 S, 5 L, 6 L and 8 L (Fig. 3a,b), whereas ‘AL Bandeirante’ displayed signals in 1 L, 2 L, 4 L, 5 S, 5 L and 8 L, also presenting heterozygosity for the presence/absence of signals in the chromosome pairs 1 and 5 (Fig. 3c,d). The karyotypes of the analyzed Z. mays accessions also differed in relation to Grande LTR–retrotransposon mapping. Uniform hybridization signals from this probe were obtained in ten metaphases of ‘Milho Pipoca Americano RS 20’ (Fig. 4a). The ‘AL Bandeirante’ karyotype exhibited stronger hybridization signals throughout the chromosome length in 15 metaphases (Fig. 4b), indicating that ‘AL Bandeirante’ possesses more copies of this LTR–retrotransposon.
180-bp sequence site mapping from probe labelled with Tetramethylrhodamine 5-dUTP (red) in metaphases of (a,b) ‘15-1149-1’ and (c,d) ‘AL Bandeirante’. (a,b) In ‘15-1149-1’, 180-bp was mapped in the interstitial portion of the long arm in chromosomes 3, 5, 6 and 8, and in the terminal portion of the short arm on chromosome 4. (a) Note the translocation in the short arm of the chromosome 1, and (b) the heterozygous terminal deletion in the chromosome 3 long arm around the large block of 180-pb sequence. (c,d) In ‘AL Bandeirante’, 180-bp sequence was mapped on in the interstitial portion of the long arm in chromosomes 1, 2, 4, 5 and 8, and in the terminal portion of the short arm in chromosome 5. Note the heterozygosity of the 180-bp sequence in the chromosomes 1 and 5. Bar = 10 µm. Images were digitized using the Image-Pro Plus software version 6.1 (https://www.mediacy.com/imageproplus).
Mapping of the Grande LTR-retrotransposon sequence, from probes labelled with Tetramethylrhodamine 5-dUTP (red), in chromosomes of (a) ‘Milho Pipoca Americano RS 20’ and (b) ‘AL Bandeirante’. (a) Hybridization signals were fully labeled in the ten chromosomes, telomere to telomere, in all metaphases of ‘Milho Pipoca Americano RS 20’. (b) In ‘AL Bandeirante’ metaphases, the chromosomes displayed strong hybridization signals. Bar = 10 µm Images were digitized using the Image-Pro Plus software version 6.1 (https://www.mediacy.com/imageproplus).
Discussion
Comparing the nuclear DNA content of Z. diploperennis (2 C = 5.76 pg), ‘Milho Pipoca Americano RS 20’ (2 C = 5.55), ‘15-1149-1’ (2 C = 2.00 pg) and ‘AL Bandeirante’ (2 C = 6.10 pg), a difference of up to 4.10 pg was found. Considering the chromosomal DNA content of ‘AL Bandeirante’, and according to mean values reported by Silva et al.15, the 4.10 pg corresponds to approximately five times the chromosome 1 (2 C = 0.80 pg = 4.00 pg) or ten times the chromosome 10 (2 C = 0.38 pg = 3.80 pg). These data reinforce the intraspecific variation in nuclear DNA content observed in Z. mays, which has been reported to range from 2 C = 4.50 pg to 7.11 pg13,14. Given this variation, karyotype differences and similarities were sought inside each Z. mays accession studied here.
Based on divergences regarding nuclear genome size, the first step was to verify the evolutionary relationship of the Z. mays spp. mays accessions. Genomic probes of Z. diploperennis provided hybridization signals, from telomere to telomere, in the chromosomes of ‘AL Bandeirante’ and ‘Milho Pipoca Americano RS 20’, confirming the genomic affinity of these accessions to the basal species Z. diploperennis1. Hybridization signals were also observed over all Z. mays ssp. mays chromosomes with the genomic probe of Z. diploperennis, but they were weaker than those detected with genomic probes of Z. mays spp. mexicana and Z. mays spp. parviglumis37, which are more phylogenetically close to Z. mays spp. mays1. Reinforcing the evolutionary relationship, the F1 hybrids of Z. diploperennis x Z. mays ssp. mays presented regular meiotic chromosome pairing and high pollen viability38,39.
The genome homology between ‘AL Bandeirante’ and ‘Milho Pipoca Americano RS 20’ was also endorsed by chromosome painting of the chromosome 1. Using the application proposed by Soares et al.40, chromosome painting between ‘AL Bandeirante’ and ‘Milho Pipoca Americano RS 20’ was informative in comparative karyotype analysis, reflecting the common evolutionary origin of these accessions. This approach has been successfully used for the resolution of phylogenetic questions in Brachypodium genus26. The GISH and chromosome painting data obtained here confirmed that popcorn belongs to Z. mays ssp. mays.
After confirming the evolutionary origin, some karyotype divergences were evidenced among the Z. mays accessions, as well as differences between homologue chromosome pairs in the same karyotype, providing cytogenetic data to understand the intraspecific variation in nuclear DNA content. Translocation and terminal deletion were distinguished in ‘15-1149-1’, which resulted in a morphological change in the chromosomes 1 and 3, respectively. Thus, cytogenetic preparations applying cell dissociation combined with air-drying technique were considered essential for the correct interpretation of these karyotype changes, since this methodology replaces the squashing step that can promote chromosome breakages41. The terminal deletion in the long arm of chromosome 3 occurred around the knob, which is considered a hotspot of chromosome structure alterations. The knobs present a complex organization, in which blocks of 180-bp sequence are interrupted by LTR–retrotransposons21. Structural chromosomal rearrangements occur within regions composed of repetitive DNA sequences24,30. In addition, Lysák and Schubert42 related that repetitive sequences scattered throughout the genome, especially TEs, are involved in various chromosomal rearrangements, such as deletion and translocation, because ectopic homologous sequences provide a template for recombination during the repair of double-strand breaks, a phenomenon denominated ectopic recombination.
The translocation and terminal deletion found in ‘15-1149-1’ represent one of the causes associated to the relatively lower nuclear DNA content (2 C = 2.00 pg) of this accession in relation to the others. In S. lycopersicum, a difference of 2 C = 0.09 pg between the wild type and the mutant ‘BHG 160’ was found to be due to a heterozygous terminal deletion in the short arm of the chromosome 143. The translocation is another karyotype aberration that includes a broken chromosome, resulting in a chromatid fragment. However, differently from a deletion or inversion, the chromatid fragment moves and joins the homologue pair or another chromosome44. Therefore, the translocation in the short arm of chromosome 1 also evidences that chromosome breakage occurred in the ‘15-1149-1’ karyotype.
These structural chromosome aberrations were highlighted in ‘15-1149-1’ and associated to low nuclear DNA content, yet other karyotype divergences were found from the mapping of the 180-bp sequence and Grande LTR-retrotransposon. Intraspecific variation in nuclear DNA content was also an outcome of the number and heterozygosity of the 180-bp sequence in the karyotype, as well as of the Grande LTR-retrotransposon signals found among the Z. mays accessions. This result shows that repetitive sequences typical of heterochromatin portions, LTR-retrotransposons and 180-bp knobs, are also responsible for genome size variation within Z. mays. The increase in nuclear genome size in this species has also been correlated with 180-bp knob abundance45. Furthermore, Bilinski et al.18 demonstrated that the variations in nuclear genome size are driven by natural selection, causing changes in the abundance of repeat sequences across the genome of Z. mays, as significant reductions in heterochromatic knobs. Knobs, which are constituted by 180-bp and 350-bp sequences, are polymorphic in relation to their number, size and distribution across the ten Z. mays chromosomes17,46, affecting 5–20% of the length of the chromosome arm47. Besides, the heterozygosity observed in the chromosome portions 1 L and 5 S only in ‘AL Bandeirante’ is also a karyotype evidence of the differential accumulation of the 180-bp sequence, and consequent change in the chromosomal DNA content among accessions. A heterozygous condition has been appointed as a cause of crossing-over suppression48. Although the origin of this polymorphism is still uncertain, it has been presumed that knobs can move around according to the “complex megatransposons” hypothesis49. This hypothesis proposes that TR-1 tandem repeat sequences are capable of forming fold-back DNA segments, driving the knobs in the Z. mays genome49.
Regarding the distribution of the Grande LTR-retrotransposon, which belongs to the Gypsy superfamily, ‘AL Bandeirante’ stood out with stronger hybridization signals than ‘Milho Pipoca Americano RS 20’. In addition to this mapping, represented by karyograms, the influence of the Grande LTR-retrotransposon sequence on nuclear DNA content among Z. mays accessions was also demonstrated. Grande LTR-retrotransposon distribution produced a uniform hybridization pattern along the extension of metaphasic Z. mays chromosomes, differently from was reported by Mroczek and Dawe10 and Lamb et al.9 that found a speckled hybridization pattern along the chromosome extension. Distribution of the Gypsy LTR-retrotransposon in different chromosome regions has also been reported for other species. In Arabidopsis thaliana (L.) Heynh50 and Asparagus officinalis L.24, this LTR is mainly distributed in the centromeres. Differently, in Silene latifolia Poir., the signals for this LTR were observed in subtelomeric heterochromatin regions of the chromosomes51.
Many mechanisms have shaped the karyotype organization in plants, such as LTR-retrotransposons8. The higher 2 C value and more Grande LTR-retrotransposon signals in ‘AL Bandeirante’ than in ‘Milho Pipoca Americano RS 20’ reflect the consequences of the LTR-retrotransposon dynamism – amplification and/or loss. Increase in nuclear genome size is promoted by a de novo DNA sequence of the retrotransposon that is inserted into the genome after an RNA intermediate to be converted into a cDNA molecule by the reverse transcriptase4,52. On the other hand, unequal and illegitimate recombination are associated with a high frequency of genomic DNA loss, and may counterbalance the amplification of LTR-retrotransposons53, as reported for Arabidopsis54 and Oryza sativa L.55.
Conclusions
Cytogenomic analysis showed that the intraspecific nuclear genome size variation in Z. mays spp. mays accessions was promoted by structural chromosome alterations and repetitive sequences. Considering the genomic relationship between the accessions, the dynamic genome of Z. mays was newly demonstrated by occurrence of terminal deletions, translocations, 180-bp knob sequence and Grande LTR-retrotransposon. Therefore, multiple karyotype factors are related to the changes in Z. mays and should be explored in other plant species.
Material and Methods
Plant material
Seeds of Z. diploperennis and Z. mays ‘15-1149-1’ (popcorn) were provided by the Maize Germplasm Bank of the Embrapa Maize and Sorghum (Sete Lagoas, Minas Gerais – Brazil) and Dr. Marcelo Soriano Viana (Universidade Federal de Viçosa, Minas Gerais – Brazil), respectively. Commercial seeds of Zea mays ‘AL Bandeirante’ and ‘Milho Pipoca Americano RS 20’ were also used. Seeds of the flow cytometry standards Solanum lycopersicum L. ‘Stupické’ and Zea mays ‘CE-777’ were provided by Dr. Jaroslav Doležel (Experimental Institute of Botany – Czech Republic). According to the Zea phylogeny proposed by Hufford et al.1 based on data from ~1000 SNPs by Fang et al.3, Z. diploperennis is basal in relation to Z. mays spp. mays. Therefore, this species was used to compare the nuclear genome size and to construct genomic probes in order to verify the ancestral relationship and homology among Z. mays spp. mays accessions.
Nuclear genome size
In order to avoid G0/G1 peak overlapping in flow cytometry histograms due to close nuclear DNA content, the nuclear genome sizes of Z. diploperennis, ‘AL Bandeirante’, ‘Milho Pipoca Americano RS 20’ and ‘15-1149-1’ (samples) were measured using the reference standards S. lycopersicum or Z. mays (2 C = 2.00 pg and 2 C = 5.55 pg, respectively; Praça-Fontes et al.56. Leaf fragments from each sample and each internal standard (S. lycopersicum or Z. mays) were co-chopped57, and the nuclei were isolated and stained using Otto buffers58, following the procedure proposed by Praça-Fontes et al.56. The nuclei suspensions were stained with propidium iodide and analyzed in a BD Accuri C6 flow cytometer (Accuri cytometers, Belgium) equipped with a laser source to detect emissions at FL3 (>670 nm). The histograms were analyzed using the BD CSampler software. Four technical replicates were performed for each sample with each standard, analyzing over 10,000 nuclei each time. The mean 2 C nuclear genome size was measured for each Zea sample by dividing the mean channel of the fluorescence peak corresponding to the standard’s G0/G1 nuclei by that of each sample.
Due to the intraspecific variation in mean 2 C value among the Z. mays accessions, the karyotypes were characterized with the aim of identifying possible differences and similarities among them. For this, the 180-bp knob sequence and Grande LTR–retrotransposon were mapped via fluorescence in situ hybridization (FISH). In addition, GISH using the genomic DNA of the wild related species Z. diploperennis was performed to confirm the evolutionary origin of popcorn. Owing to constraints in seed availability and low germination rate, ‘15-1149-1’ was replaced by ‘Milho Pipoca Americano RS 20’.
Chromosome preparation
Roots of all accessions showing 1 cm in length were incubated for 18 h in 0.20 g L−1 MS salts (Sigma) and 1.75 mM hydroxyurea (inhibitor of ribonucleotide reductase, Sigma) at 30 °C. The roots were washed in dH2O for four times of 15 min, and then treated with 3 µM amiprophos-methyl (inhibitor of microtubule polymerization, Sigma) for 4 h at 30 °C. Later, the roots were fixed in 3:1 methanol: acetic acid solution, with three changes of 10 min each, and stored at −20 °C. Afterwards, the roots were again washed for three times in dH2O, then macerated for 2 h at 36 °C in enzymatic pool (4% cellulase Sigma, 0.4% hemicellulase Sigma, 1% macerozyme Onozuka R10 Yakult, 100% pectinase Sigma) diluted in dH2O in the proportion 1:8 (enzyme pool: dH2O). After the maceration procedure, the roots were washed in dH2O, fixed in 3:1 methanol: acetic acid solution and stored at −20 °C15. From the macerated root meristems, slides were prepared by cellular dissociation and air-drying techniques41. The slides were chosen for FISH and GISH based on their number of metaphases with morphologically preserved chromosomes, exhibiting well-defined telomere and primary constriction.
Slides showing karyotypes with possible structural chromosome alterations were subjected to Feulgen reaction, a method employed to stoichiometrically and specifically stain the DNA. Thereby, as done by Silva et al.15, selected slides were immediately placed in a fixative solution of methanol: 37% formaldehyde: acetic acid (17:5:1) for 24 h at 25 °C. After fixation, the slides were washed in dH2O, air-dried, hydrolyzed in 5 M HCl for 18 min at 25 °C, and stained with Schiff’s reagent (Merck) for 16 h at 4 °C.
Genomic homology among Z. mays accessions
Genomic DNA of ‘Milho Pipoca Americano RS 20′ and Z. diploperennis were obtained according to Doyle and Doyle59. DNA concentration and purity were determined by spectrophotometry using NanoDrop (Invitrogen), and DNA integrity was further verified by 1.5% agarose gel electrophoresis. The genomic DNA was amplified and labeled by degenerate oligonucleotide-primed polymerase chain reaction (DOP-PCR). The amplification reaction mix consisted of 4 μM degenerated oligonucleotide primer (DOP 5′-CCGACTCGAGNNNNNNATGTGG-3′), 200 ng of genomic DNA of ‘Milho Pipoca Americano RS 20’ or Z. diploperennis, 200 μM of each dNTP (Promega), 1X polymerase buffer, and 2.5 U AccuTaq LA DNA Polymerase (Sigma). The labeling reaction consisted of 4 μM DOP, 200 ng of the genomic DNA, 200 μM each of dATP, dCTP and dGTP, 150 μM dTTP, 50 μM ChromaTide Alexa Fluor 488-5-dUTP (Life Technologies), 1X enzyme reaction buffer (Sigma), and 2.5 U AccuTaq LA DNA Polymerase (Sigma). The PCR conditions were: 96 °C for 3 min; 30 cycles of denaturation at 91 °C for 1 min; 56 °C for 1 min; increments of 0.1 °C/s until 68 °C; and 68 °C for 5 min. The labeled genomic probes were quantified in a NanoDrop spectrophotometer (Invitrogen) and evaluated by electrophoresis in 1.5% agarose gel. The amplified fragments ranged from 200 to 700 bp. The genomic DNA probe of Z. diploperennis was hybridized in ‘AL Bandeirante’ and ‘Milho Pipoca Americano RS 20’, and the genomic DNA probe of ‘Milho Pipoca Americano RS 20’ was hybridized in ‘AL Bandeirante’.
Recently, our research group constructed a chromosome-specific probe for the chromosome 1 of Z. mays ssp. mays ‘AL Bandeirante’40, which was used for chromosome painting in Z. mays. DNA from the chromosome 1, previously amplified by DOP-PCR as reported in Soares et al.40, was used in a new labeling reaction. The PCR program and labeling of the amplified fragment were performed as described above. The probe obtained from chromosome 1 was evaluated by electrophoresis in 1.5% agarose gel, showing fragments ranging from 100 to 900 bp.
Mapping of the 180-bp knob sequence and Grande LTR–retrotransposon
The Grande LTR–retrotransposon probe was generated by PCR using the primers F: 5′-TGCGAGGATAAGTCGGCGAAG-3′ and R: 5′-GGTGTTTTTAGGAGTAGGACGGTG-3′10. This family was selected for its wide distribution in the Z. mays genome. The probe of the 180-bp knob sequence was amplified from the primers F: 5′-ATAGCCATGAACGACCATTT-3′ and R: 5′-ACCCCACATATGTTTCCTTG-3′14. The reaction mixture consisted of: 0.5 μM of each primer, 200 ng of genomic DNA, 200 μM of each dNTP (Promega), 1X reaction buffer (Invitrogen), 2 mM MgSO4 (Invitrogen), and 2 U Platinum Taq DNA Polymerase High Fidelity (Invitrogen). The PCR conditions for the Grande LTR–retrotransposon were as follows: initial denaturation at 95 °C for 5 min; 30 cycles of denaturation at 95 °C for 1 min; annealing at 66 °C for 1 min; extension at 68 °C for 1 min and 30 sec; and final extension at 68 °C for 5 min. For the 180-bp sequence, amplification conditions were the following: initial denaturation at 95 °C for 5 min; 30 cycles of denaturation at 95 °C for 1 min; annealing at 47 °C for 1 min; extension at 68 °C for 1 min and 30 sec; and final extension at 68 °C for 5 min. The labeling reaction consisted of 0.5 μM of each primer, 200 ng of the amplified DNA, 200 μM each of dATP, dCTP and dGTP, 150 μM dTTP, 40 μM Tetramethylrhodamine 5-dUTP (Roche), 1X of the enzyme reaction buffer (Invitrogen), and 2.5 U AccuTaq LA DNA Polymerase. The PCR conditions were the same as described above for each sequence.
In situ hybridization
The procedures were performed as described by Soares et al.40 and Schwarzacher and Heslop-Harrison60, with modifications. Briefly, the slides were washed in 1X PBS buffer for 5 min, fixed with 4% formalin for 15 min, washed again in 1X PBS for 5 min, and dehydrated in cold ethanol series (70%, 85% and 100%) for 5 min each. Chromosome denaturation was carried out in 70% formamide/2X saline-sodium citrate (SSC) buffer for 3 min, at 68 °C for ‘Milho Pipoca Americano RS’ and ‘15-1149-1’ and 70 °C for ‘AL Bandeirante’. The difference in temperature is due to over-denaturation when popcorn chromosomes were submitted to 70 °C. Subsequently, the slides were dehydrated in cold ethanol series (70%, 85% and 100%). The hybridization mixture consisted of 50% formamide (Sigma) + 2X SSC (Sigma), 35 μg competitor DNA (Herring Sperm DNA, Promega) and 200 ng of the probe, with denaturation at 85 °C for 5 min followed by immediate transfer to ice. Slides were incubated with 35 μL hybridization mixture, covered by plastic coverslip HybriSlip (Sigma) and sealed with Rubber Cement (Elmer’s). The hybridization procedure was conducted in a ThermoBrite system (ThermoFisher) at 37 °C for 24 h. After this period, stringency washes were performed in three solutions of 50% formamide/2X SSC and one of 2X SSC, for 5 min each, at 42 °C for ‘Milho Pipoca Americano RS’ and ‘15-1149-1’ and 45 °C for ‘AL Bandeirante’. Metaphases were counterstained with 40% glycerol/PBS + 6-diamidino-2-phenylindole (DAPI). The same slides used for FISH mapping of the 180-bp knob sequence were also used to evaluate the differential DAPI banding pattern.
The images were captured with a digital video camera 12-bit CCD (Olympus) coupled to a photomicroscope Olympus BX-60 equipped with epifluorescence and immersion objective of 100×, numeric aperture of 1.4. The frame was digitized using the Image-Pro Plus 6.1 software (Media Cybernetics).
Change history
26 June 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Hufford, M. B., Bilinski, P., Pyhäjärvi, T. & Ross-Ibarra, J. Teosinte as a model system for population and ecological genomics. Trends Genet. 28, 606–615 (2012).
Iltis, H. H. & Doebley, J. F. Taxonomy of Zea (Gramineae). II. subspecific categories in the Zea mays complex and a generic synopsis. Am. J. Bot. 67, 994–1004 (1980).
Fang, Z. et al. Megabase-scale inversion polymorphism in the wild ancestor of maize. Genetics 191, 883–894 (2012).
Schnable, P. S. et al. The B73 maize genome: complexity, diversity, and dynamics. Science 326, 1112–1115 (2009).
Doebley, J., Goodman, M. M. & Stuber, C. W. Patterns of isozyme variation between maize and Mexican annual teosinte. Econ. Bot. 41, 234–246 (1987).
Doebley, J. Molecular evidence and the evolution of maize. Econ. Bot. 44, 6–27 (1990).
Matsuoka, Y. et al. A single domestication for maize shown by multilocus microsatellite genotyping. Proc. Natl. Acad. Sci. 99, 6080–6084 (2002).
Baucom, R. S. et al. Exceptional diversity, non-random distribution, and rapid evolution of retroelements in the B73 maize genome. PLoS Genet. 5, e1000732 (2009).
Lamb, J. C. et al. Distinct chromosomal distributions of highly repetitive sequences in maize. Chromosom. Res. 15, 33–49 (2007).
Mroczek, R. J. & Dawe, R. K. Distribution of retroelements in centromeres and neocentromeres of maize. Genetics 165, 809–819 (2003).
Sanmiguel, P. & Bennetzen, J. L. Evidence that a recent increase in maize genome size was caused by the massive amplification of intergene retrotransposons. Ann. Bot. 82, 37–44 (1998).
Roessler, K. et al. The genome-wide dynamics of purging during selfing in maize. Nat. Plants 5, 980–990 (2019).
Díez, C. M. et al. Genome size variation in wild and cultivated maize along altitudinal gradients. New Phytol. 199, 264–276 (2013).
Fourastié, M. F., Gottlieb, A. M., Poggio, L. & González, G. E. Are cytological parameters of maize landraces (Zea mays ssp. mays) adapted along an altitudinal cline? J. Plant Res. 131, 285–296 (2018).
Silva, J. C., Carvalho, C. R. & Clarindo, W. R. Updating the maize karyotype by chromosome DNA sizing. PLoS One 13, e0190428 (2018).
Poggio, L., Rosato, M., Chiavarino, A. M. & Naranjo, C. A. Genome size and environmental correlations in maize (Zea mays ssp. mays, Poaceae). Ann. Bot. 82, 107–115 (1998).
Realini, M. F., Poggio, L., Cámara Hernández, J. & González, G. E. Exploring karyotype diversity of Argentinian guaraní maize landraces: relationship among South American maize. PLoS One 13, e0198398 (2018).
Bilinski, P. et al. Parallel altitudinal clines reveal trends in adaptive evolution of genome size in Zea mays. PLoS Genet. 14, e1007162 (2018).
Mondin, M. et al. Karyotype variability in tropical maize sister inbred lines and hybrids compared with KYS standard line. Front. Plant Sci. 5, 1–12 (2014).
Peacock, W. J., Dennis, E. S., Rhoades, M. M. & Pryor, A. J. Highly repeated DNA sequence limited to knob heterochromatin in maize. Proc. Natl. Acad. Sci. 78, 4490–4494 (1981).
Ananiev, E. V., Phillips, R. L. & Rines, H. W. Complex structure of knob DNA on maize chromosome 9: retrotransposon invasion into heterochromatin. Genetics 149, 2025–2037 (1998).
Albert, P. S., Gao, Z., Danilova, T. V. & Birchler, J. A. Diversity of chromosomal karyotypes in maize and its relatives. Cytogenet. Genome Res. 129, 6–16 (2010).
McClintock, B. A cytological demonstration of the location of an interchange between two non-homologous chromosomes of Zea mays. Proc. Natl. Acad. Sci. 16, 791–796 (1930).
Li, S. F. et al. Chromosome evolution in connection with repetitive sequences and epigenetics in plants. Genes (Basel) 8, 290 (2017).
Lou, Q., Iovene, M., Spooner, D. M., Buell, C. R. & Jiang, J. Evolution of chromosome 6 of Solanum species revealed by comparative fluorescence in situ hybridization mapping. Chromosoma 119, 435–442 (2010).
Betekhtin, A., Jenkins, G. & Hasterok, R. Reconstructing the evolution of Brachypodium genomes using comparative chromosome painting. PLoS One 9, e115108 (2014).
Albert, P. S. et al. Whole-chromosome paints in maize reveal rearrangements, nuclear domains, and chromosomal relationships. Proc. Natl. Acad. Sci. 116, 1679–1685 (2019).
Schubert, I. & Lysak, M. A. Interpretation of karyotype evolution should consider chromosome structural constraints. Trends Genet. 27, 207–216 (2011).
Lysak, M. A. et al. Mechanisms of chromosome number reduction in Arabidopsis thaliana and related Brassicaceae species. Proc. Natl. Acad. Sci. 103, 5224–5229 (2006).
Schubert, I., Rieger, R., Fuchs, J. & Pich, U. Sequence organization and the mechanism of interstitial deletion clustering in a plant genome (Vicia faba). Mutat. Res. 325, 1–5 (1994).
Ziegler, K. Popcorn in Specialty Corns (ed. Hallauer, A. R.) 205–240 (CRC Press, 2001).
Zinsly, J. R. & Machado, J. A. Milho pipoca in Melhoramento e produção do milho no Brasil (ed. Paterniani, E.) 339–348 (Fundação Cargil, 1978).
Sturtevant, E. L. Notes on maize. J. Torrey. Bot. Soc. 21, 319–343 (1894).
Bailey, L. H. Manual of cultivated plants (Macmillan & Co. Ltd, 1924).
Erwin, A. T. The origin and history of pop corn, Zea mays L. var. indurata (Sturt.) Bailey mut. everta (Sturt.) Erwin. Agron. J. 41, 53–56 (1949).
Rosado, T. B., Clarindo, W. R. & Carvalho, C. R. An integrated cytogenetic, flow and image cytometry procedure used to measure the DNA content of Zea mays A and B chromosomes. Plant Sci. 176, 154–158 (2009).
González, G. E. & Poggio, L. Genomic affinities revealed by GISH suggests intergenomic restructuring between parental genomes of the paleopolyploid genus Zea. Genome 58, 433–439 (2015).
Naranjo, C. A., Molina, M. C. & Poggio, L. Evidencias de un número básico X=5 en el género Zea y su importancia en estudios del origen del maíz. Acad. Nac. Ciencias Exactas, Físicas y Nat. Buenos Aires 5, 43–53 (1990).
Poggio, L., González, G., Confalonieri, V., Comas, C. & Naranjo, C. A. The genome organization and diversification of maize and its allied species revisited: evidences from classical and FISH-GISH cytogenetic analysis. Cytogenet. Genome Res. 109, 259–267 (2005).
Soares, F. A. F. et al. Plant chromosome-specific probes by microdissection of a single chromosome: is that a reality? Front. Plant Sci. 11, 1–9 (2020).
Carvalho, C. R. & Saraiva, L. S. An air drying technique for maize chromosomes without enzymatic maceration. Biotech. Histochem. 68, 142–145 (1993).
Lysák, M. A. & Schubert, I. Mechanisms of chromosome rearrangements in Plant Genome Diversity Volume 2 (eds. Greilhuber, J., Dolezel, J. & Wendel, J. F.) 137–147 (Springer Vienna, 2013).
Karsburg, I. V., Carvalho, C. R. & Clarindo, W. R. Identification of chromosomal deficiency by flow cytometry and cytogenetics in mutant tomato (Solanum lycopersicum, Solanaceae) plants. Aust. J. Bot. 57, 444 (2009).
Burnham, C. R. Chromosomal interchanges in plants. Bot. Rev. 22, 419–552 (1956).
Jian, Y. et al. Maize (Zea mays L.) genome size indicated by 180-bp knob abundance is associated with flowering time. Sci. Rep. 7, 5954 (2017).
Xiong, Z. et al. Heterozygosity of knob-associated tandem repeats and knob instability in mitotic chromosomes of Zea (Zea mays L. and Z. diploperennis Iltis Doebley). J. Integr. Plant Biol. 47, 1345–1351 (2005).
Kato, A., Lamb, J. C. & Birchler, J. A. Chromosome painting using repetitive DNA sequences as probes for somatic chromosome identification in maize. Proc. Natl. Acad. Sci. 101, 13554–13559 (2004).
Ghaffari, R., Cannon, E. K. S., Kanizay, L. B., Lawrence, C. J. & Dawe, R. K. Maize chromosomal knobs are located in gene-dense areas and suppress local recombination. Chromosoma 122, 67–75 (2013).
Ananiev, E. V., Phillips, R. L. & Rines, H. W. A knob-associated tandem repeat in maize capable of forming fold-back DNA segments: are chromosome knobs megatransposons? Proc. Natl. Acad. Sci. 95, 10785–10790 (1998).
Initiative, T. A. G. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408, 796–815 (2000).
Kejnovsky, E. et al. Retand: a novel family of gypsy-like retrotransposons harboring an amplified tandem repeat. Mol. Genet. Genomics 276, 254–263 (2006).
Wicker, T. et al. A unified classification system for eukaryotic transposable elements. Nat. Rev. Genet. 8, 973–982 (2007).
Tenaillon, M. I., Hollister, J. D. & Gaut, B. S. A triptych of the evolution of plant transposable elements. Trends Plant Sci. 15, 471–478 (2010).
Devos, K., Brown, J. & Bennetzen, J. Genome size reduction through illegitimate recombination counteracts genome expansion in Arabidopsis. Genome Res. 12, 1075–1079 (2002).
Vitte, C., Panaud, O. & Quesneville, H. LTR retrotransposons in rice (Oryza sativa, L.): recent burst amplifications followed by rapid DNA loss. BMC Genomics 8, 218 (2007).
Praça-Fontes, M. M., Carvalho, C. R. & Clarindo, W. R. C-value reassessment of plant standards: an image cytometry approach. Plant Cell Rep. 30, 2303–2312 (2011).
Galbraith, D. W. et al. Rapid flow cytometric nalysis of the cell cycle in intact plant tissues. Science 220, 1049–1051 (1983).
Otto, F. DAPI staining of fixed cells for high-resolution flow cytometry of nuclear DNA in Methods in Cell Biology (eds. Darzynkiewiez, Z. & Crissman, H. A.) 105–110 (Academic Press, 1990).
Doyle, J. & Doyle, J. Isolation of plant DNA from fresh tissue. Focus (Madison) 12, 13–15 (1990).
Schwarzacher, T. & Heslop-Harrison, P. Practical in situ hybridization (BIOS Scientific Publishers Ltd, 2000).
Acknowledgements
The authors would like to thank the Conselho Nacional de Pesquisa (CNPq, Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) – Finance Code 001, and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil) for providing financial support to this study. We are also grateful to the Maize Germplasm Bank of Embrapa Maize and Sorghum (Minas Gerais, Brazil) and Dr. Marcelo Soriano Viana (Universidade Federal de Viçosa, Minas Gerais – Brazil) for kindly provided the Zea diploperennis and ‘15-1149-1’ (popcorn) seeds, respectively.
Author information
Authors and Affiliations
Contributions
J.C.S., F.A.F.S., M.C.S. and W.R.C. equally contributed to this work and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Silva, J.C., Soares, F.A.F., Sattler, M.C. et al. Repetitive sequences and structural chromosome alterations promote intraspecific variations in Zea mays L. karyotype. Sci Rep 10, 8866 (2020). https://doi.org/10.1038/s41598-020-65779-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-65779-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.