Abstract
Purpose
The objective of this paper is to describe the optical coherence tomography (OCT) characteristics of patients with full-thickness traumatic macular hole (TMH) and to correlate them with biomicroscopy findings.
Methods
Twelve eyes of ten consecutive patients with full-thickness TMH participated in this observational retrospective multicentre study. Patients underwent biomicroscopic fundus examination, colour fundus photography, and OCT.
Results
Traumatic macular hole was documented with OCT in five women and five men. Mean (range) time between trauma and macular hole (MH) diagnosis was 8.1 (1–24) months. The shape of TMHs was round in 11 (91.7%) eyes. The posterior vitreous was completely detached in six (50%) eyes, and with an operculum in one (8.3%) eye. The common findings seen on OCT were: (1) full-thickness loss of retinal tissue through the hole with sharp edges, perpendicular to the retinal pigment epithelium in five (41.7%) eyes; (2) TMH with an operculum totally detached from the hole's edge in two (16.7%) eyes; (3) presence of epiretinal membrane around of the hole in three (25%) eyes; and (4) presence of abnormalities of the surrounding retina in all (100%) eyes. The OCT characteristics correlated well with biomicroscopic findings, and these characteristics may be predictive for final visual acuity (VA) in TMHs. Only one of the TMHs closed spontaneously in our series.
Conclusion
Optical coherence tomography complements biomicroscopy in the evaluation of full-thickness TMHs.
Similar content being viewed by others
Introduction
Most macular holes are idiopathic and age related, however they also may be associated with myopia, trauma, scleral buckling, pneumatic retinopexy, and vitrectomy.1, 2 Historically, the first case description of traumatic macular hole (TMH) was made in 1869 by Knapp3 in a patient with ocular contusion injury. Noyes4 was the first to recognize that the hallmark of the lesion was a full-thickness defect within the centre of the macula. Traumatic macular hole formation is uncommon, usually occurs in young males, and the incidence varies in the literature from 1 to 9%.5, 6, 7, 8
The exact mechanism by which TMHs form remains subject to debate. There are many theories about their pathogenesis but not any one has sufficiently strong arguments due to the limited information available about its natural history and to the fact that they occur so infrequently.8, 9, 10, 11, 12 One of the earliest theories in the early 1900s assumed that the retinal stretching resulted from ocular deformity and/or the strong force of impact on the posterior pole.8, 9 Today, it is recognized that TMH is not only caused by direct concussion of the eyeball, but may be secondary to other causative factors such as vitreous traction.13, 14
Optical coherence tomography (OCT) is a noninvasive imaging technology that provides cross-sectional images of the retina with reproducible display of intraretinal pathology. Optical coherence tomography imaging has proved to be an important clinical tool for understanding the pathogenesis of different retinal diseases. The use of OCT has been beneficial in the diagnosis of fine changes at the retina qualitatively and quantitatively after posterior segment trauma. Optical coherence tomography has also enabled accurate and objective follow-up in these eyes.15 Furthermore, OCT has improved the understanding and management of macular hole by providing evidence of localized perifoveal, vitreous traction, intraretinal cyst, and foveal splitting. However, little information has been provided regarding OCT characterization of TMHs.
The current study provides additional information on retinal structure, describes the OCT characteristics of patients with full-thickness TMH, and correlates them with biomicroscopic findings.
Patients and methods
We reviewed the medical records and obtained follow-up information on all consecutive patients in our files with a diagnosis of a full-thickness traumatic macular hole that were visualized with the Stratus OCT between March 2003 and May 2006 at six institutions in Venezuela, Brazil, Puerto Rico, Mexico, and Colombia. Institutional Review Board/Ethics Committee approval was obtained for this study at all six institutions. All patients had clear media and good fixation to allow good quality OCT imaging. A chart review of cases that developed a full-thickness TMH included recording data on gender, laterality, age, time from symptoms, status of fellow eye, and a description of fundus biomicroscopic findings emphasizing on vitreous status and retina morphology around and inside of the hole.
Optical coherence tomography was performed using a Zeiss OCT III Instrument (Stratus OCT; Carl Zeiss, Inc. Dublin, CA, USA) with software versions 3.0 and 4.0, through a dilated pupil by a retina specialist. All six radial high-density (512 A-scans) tomograms in every OCT macular examination were reviewed for the following qualitative findings: presence or absence of epiretinal membrane (ERM), morphologic characteristics of the edges of the defect with respect to the retinal pigment epithelium (RPE) (sharp or round), presence or absence of operculum and its characteristics (attached or detached to the edge of the hole), presence or absence of nonreflective spaces within the neurosensory retina surrounding the hole (cysts), presence or absence of the region of slightly reduced optical reflectivity and increased retinal thickness evident in the region surrounding the hole (intraretinal retinal oedema), and the presence or absence of minimally reflective spaces within or beneath the neurosensory retina in the fovea (subretinal cuff of fluid). In addition, quantitative measurements of the macular hole were performed using the OCT software manual caliper-assisted tool. The following parameters were measured: the minimal inner diameter of the hole measured at the part of neural epithelial defect in the macula using horizontal scanning; the maximal outer diameter of the hole measured at the widest distance at the bottom of the hole; and the maximal thickness or height of the margins of the hole. This evaluation of the diameter of the hole required numerous scans to ensure that the linear scan was taken exactly across the diameter of the hole, and not across the chord of the arc of the circle. If an eye did not have all the OCT information required, the patient was brought back to the clinic to obtain the complete OCT measurements described here.
The diagnosis of TMH was performed using biomicroscopy through a standard dilated retinal examination and a 78 or 66 D non-contact slit-lamp lens (Volk, Optical Inc, Mentor, OH, USA). Optical coherence tomography imaging was performed on 12 eyes of 10 patients with different stages of TMH. All cases were staged according to the classification scheme suggested by Gass13 for idiopathic macular holes.
Results
Ten patients (12 eyes) with full-thickness TMH participated in our study. The mean age of our patients was 53.7 (range: 9–62) years old. Five (50%) patients were female. Two patients had bilateral TMHs. The mean time between trauma and initial symptoms was 5.9 (range: 1–20) months. The mean time between trauma and TMH diagnosis was 8.2 (range: 1–24) months. The most common cause of TMH in our series was eye contusion in eight (66.6%) eyes. The hole was stage 2 in two (16.7%) eyes, stage 3 in four (33.3%) eyes, and stage 4 in six (50%) eyes (Gass' classification) (Tables 1, and 2).
Biomicroscopic examination showed that the shape of the macular hole was round in eleven (91.7%) eyes and elliptic in one (8.3%) eye. Three (25%) eyes had yellow deposits inside the macular hole. The posterior vitreous was completely detached in six (50%) eyes and with an operculum in one (8.3%) eye by biomicroscopic fundus examination and confirmed by OCT. In all eyes, OCT examination showed a full-thickness macular hole. There was a presence of an epiretinal membrane around the macular hole in three (25%) eyes (in these eyes, time between trauma and TMH diagnosis had a mean of 9.3 months). Morphologic characteristics of the edges of the defect with respect to the RPE showed full-thickness loss of retinal tissue through the macular hole with sharp edges perpendicular to the RPE in five (41.7%) eyes and all these eyes had a period of time between trauma and TMH diagnosis less than 3 months (Table 2). An operculum was present in three (25%) eyes and was totally detached from the macular hole's edge in two eyes (Figure 1).
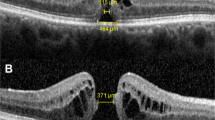
A 62-year-old woman was examined 3 months after blunt ocular trauma to her left eye. (a) Vertical optical coherence tomography (OCT) scan showed a full-thickness defect in the fovea. There was an operculum attached to one edge of the hole consistent with stage 2. The edges of the hole were sharp and thickened with areas of reduced optical reflectivity (cysts) (arrows). (b) Horizontal OCT scan. Note that the retinal operculum remained attached to the vitreous (arrowheads). The minimal inner diameter of this hole measured 299 μm, while the maximum outer diameter measured 399 μm. The height of the hole reached a maximum of 311 μm.
The presence of abnormalities of the surrounding retina was seen on OCT in 100% of eyes including nonreflective spaces within the neurosensory retina surrounding the hole (cysts) in six (50%) eyes; a region of slightly reduced optical reflectivity and increased retinal thickness evident in the region surrounding the hole (intraretinal retinal oedema) in six (50%) eyes; and minimally reflective spaces within or beneath the neurosensory retina in the fovea (subretinal cuff of fluid) in nine (75%) eyes (Table 2) (Figure 2).
Macular hole after blunt-ocular trauma (TMH). A 12-year-old boy was examined 12 months after blunt-ocular trauma to his right eye. Fundus examination showed a full-thickness TMH and posterior vitreous detachment. Optical coherence tomography (OCT) scan confirmed a full-thickness loss of retinal tissue in the fovea. The minimal inner diameter of this hole measures 1097 μm, while the maximum outer diameter measures 1596 μm. Note that the edges of the hole are round and slightly thickened, with areas of no optical reflectivity. In addition, minimally reflective spaces within or beneath the neurosensory retina in the fovea can be seen in the right edge (subretinal cuff of fluid) (arrow). The height of the hole reached a maximum of 340 μm.
The size of the hole measured on OCT was on average 634 μm (range: 99–1 569 μm) for the minimal inner diameter, and 760 μm (range: 110–1 696 μm) for the maximum outer diameter. The height of the hole had a mean of 252 μm (range: 251–347 μm). Only in one case spontaneous resolution with a total vitreous detachment was documented with OCT (Figure 3).
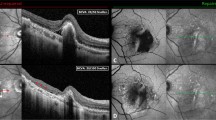
Spontaneous resolution of traumatic macular hole (TMH) documented with optical coherence tomography (OCT). (a) Colour fundus photographs and OCT scan obtained 72 h after trauma demonstrated a lamellar hole with borders with a sharp-cut edge. Note marked hyperreflectivity of the perifoveal retina associated with avulsion of part of the inner foveal retina (arrows). (b) One month after injury OCT, the lesion had increased in size demonstrating a full-thickness TMH, the borders were less sharp and nonreflective spaces (cysts) had appeared within the neurosensory retina surrounding the hole. (c and d) Two and three months after injury, the TMH had diminished in size and height. (e) Five months after injury, OCT demonstrated restoration of the foveal contour with closure of the macular hole. A foveal cyst, evident as a hiporeflective black circle, is visible just anterior to the hyporeflective band corresponding to the retinal pigment epithelium/coriocapillaris. (f) Ten months after injury, OCT revealed a closed macular hole with a nonreflective space within the neurosensory retina.
Discussion
A traumatic macular hole is a full-thickness defect of the retinal tissue involving the anatomical fovea of the eye that occurs after ocular trauma. Despite accumulating knowledge regarding the pathogenesis, natural history, and the treatment of idiopathic macular holes, little is known about the clinical and OCT characteristics of TMHs because they occur infrequently. This study of 12 eyes demonstrates that full-thickness TMHs do not have a homogeneous pattern but instead display different characteristics of the vitreous and surrounding retina based on OCT examination.
The exact mechanism of formation of traumatic macular holes is uncertain and in the setting of TMHs, the vitreous is believed to have an important role in the pathogenesis. In our study, we showed that the posterior vitreous was completely detached, commonly known as PVD, in 50% of eyes and the shape of the macular hole was round in 91.7% of eyes. This is in disagreement with previous series where Yanagiya et al16 observed that the appearance of most TMHs was elliptic in 95% of eyes and DVP was present in 15% of eyes. In addition, Johnson et al17 observed in 21 (84%) eyes, an attached posterior vitreous at the time of vitrectomy for the treatment of TMH. They theorized that the force of the impact transmitted to the macula results in rupture of the fovea. In support of this theory, they observed that the appearance of most TMHs in their experience was elliptic and not round. However, our series revealed that the appearance of most TMHs was round. The reason for this morphological difference between these studies is unknown, however 50% of our cases had a complete PVD and all eyes were quantitatively and qualitatively analysed with OCT.
Gass13 described the idiopathic macular hole classification based on pathogenesis and biomicroscopic examination. Considering that there is no classification for TMHs described in the literature, we decided to use Gass' classification for idiopathic macular hole in the current study because of its practical aspects of anatomical stage differentiation. This is particularly important for allowing the comparison of previously published reports and future studies. In addition, vitreous qualitative OCT findings and quantitative minimum inner-diameter OCT measurement aids in the classification into each stage.
Our study demonstrated that the morphology of the edges of the defect depended on the time between trauma and TMH diagnosis. Sharp edges perpendicular to the RPE tend to appear in holes with a time from trauma to TMH diagnosis under 3 months, and round edges after 3 months. Ripandelli et al18 have reported the morphological evolution of the macular hole edges from a sharp to a round contour in full-thickness idiopathic macular holes by OCT. Therefore, OCT may help in the morphological evaluation of full-thickness TMH, and in the detection of changes over time. Our findings support the hypothesis that a TMH might have occurred primarily by an extraordinary impact and secondarily, the tear matured into ‘typical’ macular hole receiving some modification from the retinal oedema, vitreous traction, or some other factors.19
The visual loss associated with a full-thickness idiopathic macular hole is attributable most likely to both the direct loss of retinal tissue, and the surrounding area of retinal detachment and thickening.20 We demonstrated the presence of abnormalities of the surrounding retina on OCT in 100% of eyes, including nonreflective spaces within the neurosensory retina surrounding the hole, a region of slightly reduced optical reflectivity and increased retinal thickness evident in the region surrounding the hole; and minimally reflective spaces within or beneath the neurosensory retina in the fovea. We suspect that these characteristics of the surrounding retina that were evident on OCT may be predictive for final visual acuity (VA) in TMHs.
Only one of our cases closed spontaneously. However, Yamashita et al21 reported spontaneous closure of TMH in 44.4% (8 eyes) of 18 patients. They revealed in three cases that were examined with OCT two distinct abnormalities; acute foveal dehiscence without the involvement of the posterior vitreous cortex, and vitreous detachment with residual vitreous adhesion to the edge of updrawn fovea that developed the release of the foveal adhesion at the time of hole closure. Our case did not show any of these OCT findings. We found acute foveal dehiscence with total vitreous detachment documented by OCT. In addition, we found marked hyperreflectivity of the perifoveal retina, which could be interpreted as an alteration that may have indeed resulted from the strong force of impact on the posterior pole associated with avulsion of part of the inner-foveal layers.
Our study is limited by its retrospective nature, and the small number of patients, which does not allow quantitative, statistical correlation between the clinical and OCT features in TMHs. However, OCT seems to be an important adjunctive tool in TMHs by enabling diagnosis of subtle changes that are essentially invisible to the examiner's eyes. It may also determine relatively mild quantitative changes that may indicate improvement or worsening in the natural course of TMHs, and that may potentially influence in decision-making regarding timing and correct therapeutic approach of these abnormalities.
In summary, OCT complements biomicroscopy in the evaluation of full-thickness TMHs. Optical coherence tomography findings and abnormalities of the surrounding retina may be important predictors of VA after surgery but further studies are necessary to confirm our observations.
References
Smiddy WE . Atypical presentations of macular holes. Arch Ophthalmol 1993; 111: 626–631.
Kokame GT . Early stage of macular hole in a severely myopic eye. Am J Ophthalmol 1995; 119: 240–242.
Knapp H . Ueber Isolirte Zerreissungen der Aderhaut infolge von Traumen auf Augapel. Arch Augenheilkd 1869; 1: 6–29.
Noyes HD . Detachment of the retina, with laceration at the macula lutea. Trans Am Ophthalmol Soc 1871; 1: 128–129.
Margherio RR, Schepens CL . Macular breaks: I diagnosis, etiology, and observations. Am J Ophthalmol 1972; 74: 219–232.
Cox MS, Schepens CL, Freeman HM . Retinal detachment due to ocular contusion. Arch Ophthalmol 1966; 76: 678–685.
Aaberg TM, Blair CJ, Gass DJD . Macular holes. Am J Ophthalmol 1970; 69: 555–562.
Aaberg TM . Macular holes: a review. Surv Ophthalmol 1970; 15: 139–162.
Duke-Elder S . System of ophthalmology. vol 14. Injuries. Henry Kimpton: London, 1972; 169–171.
Yokotsuka K, Kishi S, Tobe K, Kamei Y . Clinical features of traumatic macular hole. Jpn J Clin Ophthalmol 1991; 45: 1121–1124.
Gass JD . Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment, 4th ed. Mosby: St. Louis, 1997; 2–744.
Chuang LH, Lai CC, Yang KJ, Chen TI, Ku WC . A traumatic macular hole secondary to a high-energy Nd:YAG laser. Ophthal Surg Lasers 2001; 32: 73–76.
Gass JDM . Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment, 4th ed. Mosby: St. Louis, 1997; 904–937.
Schepens CL . Changes caused by alterations of the vitreous body. Am J Ophthalmol 1955; 39: 631–633.
Rumelt S, Karatas M, Ophir A . Potential applications of optical coherence tomography in posterior segment trauma. Ophthal Surg Lasers Imaging 2005; 36: 315–322.
Yanagiya N, Akiba J, Takahashi M, Shimizu A, Kakehashi A, Kado M et al Clinical characteristics of traumatic macular holes. Jpn J Ophthalmol 1996; 40: 544–547.
Johnson RN, McDonald HR, Lewis H, Grand MG, Murray TG, Mieler WF et al Macular hole: observations, pathogenesis, and results of vitrectomy surgery. Ophthalmology 2001; 108: 853–857.
Ripandelli G, Coppe AM, Bonini S, Giannini R, Curci S, Costi E et al Morphological evaluation of full-thickness idiopathic macular holes by optical coherence tomography. Eur J Ophthalmol 1999; 9: 212–216.
Yamada H, Sakai A, Yamada E, Nishimura T, Matsumura M . Spontaneous Closure of traumatic macular hole. Am J Ophthalmol 2002; 134: 340–347.
Sjaarda RN, Frank DA, Glaser BM, Thompson JT, Murphy RP . Assessment of vision in idiopathic macular holes with macular microperimetry using the scanning laser ophthalmoscope. Ophthalmology 1993; 100: 1513–1518.
Yamashita T, Uemara A, Uchino E, Doi N, Ohba N . Closure of traumatic macular hole. Am J Ophthalmol 2002; 133: 230–235.
Acknowledgements
Supported in part by the Arevalo-Coutinho Foundation for Research in Ophthalmology, Caracas, Venezuela.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have no financial or proprietary interest in any of the products or techniques mentioned in this article Presented in part at the 7th World Meeting of the International Society of Ocular Trauma (ISOT), Rome, Italy, June 2006
Rights and permissions
About this article
Cite this article
Arevalo, J., Sanchez, J., Costa, R. et al. Optical coherence tomography characteristics of full-thickness traumatic macular holes. Eye 22, 1436–1441 (2008). https://doi.org/10.1038/sj.eye.6702975
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702975
Keywords
This article is cited by
-
Cell composition at the vitreomacular interface in traumatic macular holes
Graefe's Archive for Clinical and Experimental Ophthalmology (2022)
-
Comparison of full-thickness traumatic macular holes and idiopathic macular holes by optical coherence tomography
Graefe's Archive for Clinical and Experimental Ophthalmology (2010)