Abstract
Background/aims
The purpose of this study was to investigate the relationship between vitreous leptin levels and retinal diseases.
Methods
Levels of vitreous leptin were evaluated in proliferative diabetic retinopathy (PDR) and a variety of other retinopathies including: macular disease, neovascular maculopathies, primary retinal detachments, and vascular occlusive disease.
Results
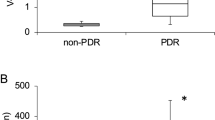
In patients with PDR (N=7), the average vitreous level of leptin (37.4 ng/ml) was significantly higher than that in patients with PVR (<1.0 ng/ml, P<0.05). Vitreous leptin level in patients with PVR or macular disease (N=18), with or without diabetes, was not significantly different from the control subjects who had retinal detachment only (N=7).
Conclusion
The results show that the leptin level in vitreous taps is elevated in PDR. We suggest that leptin plays an active role in PDR.
Similar content being viewed by others
Introduction
Retinal neovascularization is driven by a variety of angiogenic factors such as vascular endothelial growth factor (VEGF), insulin-like growth factor I (IGF-I), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), interleukins, and transforming growth factor β (TGF-β). In addition to these well-known factors, a number of novel angiogenic proteins have recently been described.1, 2, 3, 4, 5
Leptin, an antiobesity hormone, is a 167 amino-acid protein synthesized in the pituitary gland, adipose tissue, and fibroblasts in the setting of high intracellular triglycerides. Serum leptin concentrations are proportional to the body's adipose mass. Leptin induces lipid metabolism, energy use, and satiety. Receptors for leptin have been identified on endothelial cells, fibroblasts, and in the retinal pigment epithelium. Recent studies have shown leptin to act as an angiogenic factor in vivo and can cause endothelial cells to proliferate and migrate.6 It is also found in higher concentrations in the plasma of individuals with hypertensive and diabetic retinopathy.6, 9 Leptin levels in blood serum and vitreous have been correlated with neovascular eye diseases such as proliferative diabetic retinopathy.7, 11
Proliferative diabetic retinopathy (PDR) remains one of the major causes of acquired blindness in developed nations. Abnormal angiogenesis, the hallmark of PDR, leads to severe vision loss through secondary vitreous haemorrhage and/or vascular fibrosis with tractional retinal detachment. Factors and cytokines thought to play a role in the pathophysiology of PDR include: VEGF, acidic and basic FGF, TGF-α and TGF-β, and interleukins. Vitreous leptin levels in diabetic retinopathy have been shown to be elevated in patients with proliferative diabetic retinopathy.8 This same study also demonstrated elevated levels of leptin in the vitreous of subjects with primary retinal detachment. These findings were of interest to us given the lack of an obvious biologic mechanism that would explain the expression of leptin in both conditions.
Our primary goal for the present study was to evaluate vitreous leptin levels in a variety of retinal conditions.
Patients and methods
The purpose of this study was to investigate the relationship between vitreous leptin levels and retinal diseases. Ethical approval was obtained prior to the initiation of this project from the Vancouver Hospital and University of British Columbia's Institutional Review Board. The UBC CREB policies comply with the Tri Council Policy and the Good Clinical Practice Guidelines, which have their origins in the ethical principles in the Declaration of Helsinki. Written informed consent was obtained from patients. Levels of vitreous leptin were evaluated in a variety of retinopathies including: proliferative diabetic retinopathy (N=7), macular disease (N=10, macular holes, epiretinal membranes), neovascular maculopathies (N=2, age-related macular degeneration, pseudo-xanthoma elasticum with angioid streaks), primary retinal detachments (N=7), and retinal detachment with PVR (N=6), (see Table 1). All subjects underwent a standard three-port vitrectomy, with a core sample of vitreous (1.0–1.5 ml) taken prior to initiating the pars plana infusion. The undiluted vitreous samples were immediately frozen and stored at −20°C. Immunoreactive levels of leptin were determined by enzyme-linked immunoabsorbent assays (ELISA Cayman Chemical, MI, USA). (ELISA: Range 1–50 ng/ml) Duplicate assays were performed on all samples.
Results
A total of 32 samples of vitreous were taken from 32 subjects. Seven eyes had proliferative diabetic retinopathy, 12 eyes had structural macular pathology (macular holes, epiretinal membranes, vitreo-macular traction syndrome, and submacular choroidal neovascularization), six had proliferative vitreoretinopathy (full range of grades represented), seven had primary rhegmatogenous retinal detachments (see Table 1).
Of the 12 individuals with structural macular pathology, two had type II diabetes, but none of these individuals had retinopathy of greater than ETDRS grade 43. Leptin levels for all of the vitreous samples are presented in Table 1. The only study group that had elevated leptin levels beyond 1.2 ng/ml was the group with proliferative diabetic retinopathy. Two individuals who did not have PDR had leptin levels of 1.05 and 1.2; the former had a chronic total retinal detachment and the latter a massive subretinal haemorrhage secondary to age-related macular degeneration. No individuals with primary rhegmatogenous retinal detachment or structural macular pathology (diabetic or otherwise) had a vitreous leptin level above 1.00 mg/ml. One individual with diabetic retinopathy and a vitreous haemorrhage had a vitreous leptin level of <1.00 ng/ml. This person was thought to have proliferative diabetic retinopathy preoperatively; however, intraoperatively it was noted that a single focus of vitreous traction on a venous loop was the underlying pathologic mechanism that was responsible for the haemorrhage.
Discussion
Elevated vitreous leptin levels were found only in eyes with proliferative diabetic retinopathy that had some degree of recent vitreous haemorrhage. All other retinal conditions did not demonstrate significant vitreous leptin levels.
Two prior studies have evaluated vitreous leptin levels in retinal disease; the results were conflicting. In Gariano's et al7 paper from 2000, elevated vitreous leptin levels were detected in the vitreous humor of human subjects with proliferative diabetic retinopathy and retinal detachment. A subsequent paper in 2004 did not demonstrate any association between vitreous leptin levels and proliferative diabetic retinopathy.10
Our results corroborate the findings associated with proliferative diabetic retinopathy in Gariano's study, but are divergent for the retinal detachment results. Gariano et al suggested that elevated leptin levels in the setting of retinal detachment were likely related to retinal ischemia; however, methodologic differences between the present study and that of Gariano et al may have led to these different results. Specific discrepancies include our use of an ELISA technique for determining leptin levels (compared with a radioimmunoassay) and our duplication of all leptin tissue level determinations (vs a single evaluation). The present study's significantly higher vitreous leptin levels in the presence of PDR are likely related to the use of the ELISA procedure. We believe our finding of normal vitreous leptin levels in PVR and rhegmatogenous RD is a more understandable result. Hernandez et al's10 study failed to show an elevated vitreous level in 25 consecutive patients undergoing vitrectomy surgery for proliferative diabetic retinopathy; however, their study excluded subjects with vitreous haemorrhage. All of our vitreous samples that were positive for leptin had significant, recent vitreous haemorrhages. Our two samples from diabetic subjects that demonstrated normal leptin levels did not have recent vitreous bleeds (one subject had a sub-hyaloid haemorrhage and the other a had chronic ochre haemorrhage).
Our data suggest that, in eyes with PDR, leptin reaches the vitreous (a) via the extravasation of blood from active retinal neovascularization and (b) by the expression of leptin by fibroblasts11 within diabetic neovascular membranes (unpublished observations). Once in the vitreous, leptin is a likely contributor to progressive fibrovascular proliferation. As a result, strategies targeting leptin, or its receptor, may offer potential therapies for ocular angiogenesis in the setting of proliferative diabetic retinopathy.
References
Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH et al. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev 2003; 19 (6): 442–455.
Erb MH, Sioulis CE, Kuppermann BD, Osann K, Wong CG . Differential retinal angiogenic response to sustained intravitreal release of VEGF and bFGF in different pigmented rabbit breeds. Curr Ey eRes 2002; 24 (4): 245–252.
Castellon R, Hamdi HK, Sacerio I, Aoki AM, Kenney MC, Ljubimov AV . Effects of angiogenic growth factor combinations on retinal endothelial cells. Exp Eye Ees 2002; 74 (4): 523–535.
Elner SG, Elner VM, Jaffe GJ, Stuart A, Kunkel SL, Strieter RM . Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res 1995; 14 (11): 1045–1053.
Kojima S, Yamada T, Tamai M . Quantitative analysis of interleukin-6 in vitreous from patients with proliferative vitreoretinal diseases. Jpn J Ophthalmol 2001; 45 (1): 40–45.
Sierra-Honingmann MR, Nath AK, Murakami C, Garcia-Cardeña, Papapetropoulos A, Sessa WC et al. Biological action of leptin as an angiogenic factor. Science 1998; 281: 1683–1686.
Gariano RF, Nath AK, D'Amico DJ, Lee T, Sierra-Honigmann MR . Elevation of leptin in diabetic retinopathy and retinal detachment. Invest Ophthalmol Vis Sci 2000; 41: 3576–3581.
Gokhan U, Metin O, Zeki B, Vedat E, Necati B, Ozdermir IC . Is leptin associated with diabetic retinopathy. Diabetes Care 2000; 23: 373–384.
Gokhan U, Metin O, Alper S, Can K, Tayfun E, Necati B et al. Is leptin associated with hypertensive retinopathy? J Clin Endocrinol Metab 2000; 85: 683–687.
Hernandez C, Lecube A, Castellanos JM, Segura RM, Garat M, Garcia-Arumi J et al. Intravitreous leptin concentrations in patients with proliferative diabetic retinopathy. Retina 2004; 24 (1): 30–35.
Glasow A, Kiess W, Anderegg U, Berthold A, Bottner A, Kratzsch J . Expression of leptin (Ob) and leptin receptor (Ob-R) in human fibroblasts: regulation of leptin secretion by insulin. J Clin Endocrinol Metab 2001; 86 (9): 4472–4479.
Acknowledgements
The study was funded in part by a grant from Canadian Institutes of Health Research MOP 42389.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maberley, D., Cui, J. & Matsubara, J. Vitreous leptin levels in retinal disease. Eye 20, 801–804 (2006). https://doi.org/10.1038/sj.eye.6702011
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702011