Abstract
Purpose
To evaluate the long-term efficacy and safety of infliximab as treatment for noninfectious posterior uveitis.
Methods
An open-label clinical trial including seven patients (12 eyes) with posterior uveitis refractory to conventional treatment regimens with corticosteroids and at least one immunosuppressive agent. Three intravenous doses of 5 mg/kg of infliximab were administered at weeks 0, 2, and 6. Infliximab infusion was repeated in patients undergoing a relapse of uveitis after initial remission. Improvement was defined as amelioration of visual acuity or disappearance of retinal exudates and/or haemorrhages, decreased macular oedema and/or vitreous opacities. All patients were followed up for at least 36 months.
Results
Six of the seven patients (five diagnosed with Behçet's disease and one diagnosed with sarcoidosis) showed a significant improvement after the first infliximab dose. Only in one patient diagnosed with chronic idiopathic multifocal choroiditis did the drug have no effect, and this patient was withdrawn from the study. At the end of follow-up, one eye had lost one line of vision and three eyes showed improved vision. All eyes had improved in terms of signs of inflammation. No adverse effects of treatment were observed.
Conclusion
Infliximab is efficient and safe for the long-term management of refractory posterior uveitis, especially in patients with predominant retinal vasculitis and vitritis.
Similar content being viewed by others
Introduction
Noninfectious posterior uveitis belongs to a complex group of diseases in which there is an underlying immune system disorder. For years, the treatment of choice for this type of uveitis has been systemic immunosuppression with steroids or immunosuppressive agents such as cyclosporin A, azathioprine, methotrexate, cyclophosphamide, chlorambucil, or tacrolimus. However, in a large number of patients relapses occur despite aggressive treatment with these drugs, and this can lead to a significant, or even permanent, loss of vision.
The cytokine tumour necrosis factor α (TNFα) is known to actively participate in the pathogenesis of some types of uveitis. When TNFα is injected into the vitreous of rabbit eyes, it gives rise to an acute uveitis that rapidly progresses to the posterior segment. TNFα and IL-1β also seem responsible for the rapid infiltration of leukocytes and protein leakage observed in lipopolysaccharide-induced uveitis in the rabbit.1, 2, 3 These and other findings have made TNFα a potential target for immunotherapy in this type of disease, and the administration of anti-TNFα monoclonal antibodies has been related to a significant improvement in uveitis in some experimental models.4 In humans, anti-TNFα antibodies have proved useful for the treatment of panuveitis associated with Behçet's disease.5
The chimaeric anti-TNFα monoclonal antibody infliximab (Remicade, Schering-Plough BV, Maarsen, The Netherlands) is currently used in the treatment of autoimmune diseases such as rheumatoid arthritis,6 Crohn's disease,7 or ankylosing spondylitis.8 Moreover, its use for the short-term treatment of refractory posterior uveitis has been recently reported.9 The present prospective study, performed on a cohort of patients subjected to long-term follow-up, was designed to assess the efficiency and safety of infliximab in the treatment of this type of uveitis.
Methods
The study population was comprised of seven consecutive patients with severe, noninfectious chronic posterior uveitis resistant to the usual immunosuppressive treatment. All patients had been treated for at least 1 year with systemic steroids coadministered with one or several conventional immunosuppressants. To be enrolled in this study, the patients were required to have undergone recurrence of the disease despite maximum tolerated immunosuppressant doses or to have shown signs of systemic toxicity in response to therapy.
Informed written consent to participate in our study, which was approved by our institution's Ethics and Clinical Trials Committee, was obtained from each patient. A negative pregnancy test and acceptable form of birth control were additional requirements for female patients. Chest X-rays were taken and patients were investigated for positive tuberculin test.
Treatment was administered on a compassionate-use basis. The first infusions were administered in October of 2000. All immunosuppressive agents were discontinued 1 month before treatment initiation. The dose of prednisone was then adjusted to 0.5 mg/kg/day in every patient. After a wash-out period of 1 month, all patients were treated with three intravenous infusions of 5 mg/kg infliximab at weeks 0, 2, and 6. The prednisone dose was reduced every week by 5 mg if there was no evidence of posterior pole inflammation. Infliximab infusion was repeated in patients undergoing a relapse of uveitis after the initial remission.
All patients were evaluated by two independent ophthalmologists from baseline to the end of follow-up (36 months). Clinical visits included a complete ophthalmologic examination (best-corrected visual acuity using the standardized Snellen chart, slit-lamp examination, applanation tonometry, and ophthalmoscopy) and clinical assessments were performed at baseline and week 1, and at months 1, 4, 6, 12, 24, and 36. Fluorescein angiograms (FAG) were performed at baseline and at months 1, 4, 12, 24, and 36.
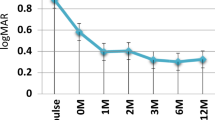
The primary outcome measure was improved uveitis. An improvement in the disease was defined as increased best-corrected visual acuity or an objective, significant improvement in posterior uveitis in terms of cessation of retinal exudates and/or haemorrhages, and reduced macular oedema and/or vitreous opacities (Nussenblatt scale10).
Results
In all, 12 eyes of seven patients were included in the study. Table 1 shows the general characteristics of patients and prior immunosuppressive therapy.
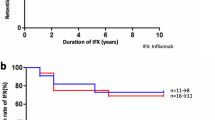
Five patients diagnosed with Behçet's disease had predominant retinal vasculitis and vitritis; one patient had sarcoidosis (diagnosed by skin biopsy confirmation, noncaseating granuloma), also with predominant retinal vasculitis and vitreous haze, and the remaining patient had idiopathic multifocal choroiditis with predominant chorioretinitis. Before enrolment in the study, all the patients had been treated with immunosuppressive drugs for a period of time ranging from 16 to 97 months. Following the three initial infliximab infusions, uveitis was markedly improved in six of the seven patients (Figures 1 and 2). Patient 7, diagnosed with idiopathic multifocal choroiditis showed no response to treatment, and was withdrawn from the study and treatment discontinued. The remaining patients showed significantly improved visual acuity and vitreous haze after the first three doses. No changes were observed as for the dissipation of haemorrhages. Since this initial remission in the six patients, five have suffered episodes of exacerbated uveitis during the 3 years of treatment requiring additional infliximab injections (Table 2).
As a result of frequent relapses in Patient 4, after the eighth infusion, it was decided to administer the drug every 8 weeks in accordance with the treatment regimen normally used in the management of rheumatoid arthritis. This patient remained asymptomatic until the end of follow-up.
At the end of the 3 years of follow-up, three out of the 10 eyes remaining in the study showed improved visual acuity, six remained the same, and only one eye lost one line of vision (Table 3).
Vitreous haze was improved with respect to pretreatment haze. During follow-up, no patient suffered worsening of extraocular symptoms or adverse effects related to treatment.
At the end of the patient's follow-up, none of them were being treated with additional doses of steroids or immunosuppressants.
Discussion
Posterior uveitis is often the consequence of an array of systemic diseases, many of which are of an autoimmune origin. Steroids, or immunosuppressants such as cyclosporin A, azathioprine, or methotrexate, have proved useful in the management of this disease. Despite adequate treatment, however, many patients with posterior uveitis experience relapses related to significant morbidity, including an irreversible loss of visual acuity in a high proportion of these patients.
Infliximab is a monoclonal antibody of proven efficacy in the treatment of some autoimmune diseases. Recently, its use for the treatment of the eye pathologies associated with these diseases has also been reported.11
Sfikakis et al5 assessed the short-term efficacy of infliximab for the treatment of sight-threatening panuveitis in Behçet's disease, treating five patients with a single dose. Inflammation was resolved in all five patients earlier than 1 week after the injection of the drug. Joseph et al9 also demonstrated the efficiency of infliximab in five patients with posterior uveitis (three with Behçet's and two with idiopathic disease) during 6 months of follow-up using a treatment regimen consisting of three 5 mg/kg doses given on day 0, and then at weeks 2 and 6.
Herein, we used this treatment regimen in a group of patients with posterior uveitis resistant to conventional immunosuppressive treatment. The follow-up period to which these patients have been subjected is currently 3 years, confirming the efficacy and safety of long-term infliximab treatment.
Six out of seven patients showed a good initial response to treatment, their clinical symptoms and visual acuity improving. Since treatment, five patients have needed further doses to control worsening of ocular symptoms. The drug adequately preserved the vision of these patients. Only one of the eyes included in our study had lost one line of vision by the end of follow-up. We thought that none of the improvements in VA were related with long-standing macular oedema in those eyes with lower visual acuities. However, a significant improvement both in fluorescein angiography and vitreous haze was observed in all subjects, what justifies the slight improvement in visual acuity observed in some of the studied eyes. Unlike the case in other published studies, immunosuppressive treatment was discontinued in all the patients 1 month before the start of treatment, requiring the use of steroids at anti-inflammatory doses, which were subsequently tapered during the course of follow-up. This enabled us to evaluate the safety of the drug, precluding the possibility of undesirable effects attributable to adjuvant immunosuppressive treatment.
Treatment with infliximab has been related to severe, albeit infrequent, detrimental side-effects occurring both at the local and systemic level. Reported ocular complications include isolated cases of bilateral anterior optic neuropathy,12 retrobulbar optic neuritis,13 and infection of the eyelids by molluscum contagiosum.14 At the systemic level, infliximab treatment has been associated with opportunistic infections (tuberculosis,9, 15 cryptococcosis,16 pneumocystis17), blood disorders (leukaemia,18 pancytopenia19), and skin disorders including neoplasias.20
During 3 years of follow-up, we observed no local or systemic secondary effects associated with infliximab treatment, and patients complained of no further extraocular symptoms in the context of their disease. On the contrary, in the patient with sarcoidosis, subcutaneous granulomas in the face and throat notably improved, in accordance with reports by other authors.21
In summary, our findings indicate that the monoclonal antibody infliximab is safe and efficient for the long-term treatment of posterior uveitis refractory to conventional immunosuppressive therapy, stabilizing the course of the illness and maintaining visual acuities. Preliminary treatment with three 5 mg/kg injections serves to control ocular inflammation, although relapses requiring further doses are common during the course of follow-up.
References
Rosenbaum JT, Simples JR, Hefeneider SH, Howes Jr EL . Ocular inflammatory effects of intravitreal interleukin 1. Arch Ophthalmol 1987; 105: 1117–1120.
Rosenbaum JT, Howes Jr EL, Rubin RM, Samples JR . Ocular inflammatory effects of intravitreally-injected tumor necrosis factor. Am J Pathol 1988; 133: 47–53.
Feisher LN, Ferrel JB, McGahan MC . Ocular inflammatory effects of intravitreally injected tumor necrosis factor-alpha and endotoxin. Inflammation 1990; 14: 325–335.
Sartani G, Silver PB, Rizzo LV, Chan CC, Wigert B, Mastorakos G et al. Anti-tumor necrosis factor alpha therapy suppresses the induction of experimental autoimmune uveoretinitis in mice by inhibiting an antigen priming. Invest Ophthalmol Vis Sci 1996; 37: 2211–2218.
Sfikakis PP, Theodossiadis PG, Katsiari CG, Kaklamanis P, Markomichelakis NN . Effect of infliximab on sight-threatening panuveitis in Behcet's disease. Lancet 2001; 358: 295–296.
Lipsky PE, Van der Heijde DM, St Clair EW, Furst SE, Breedveld C, Kalden JR et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study group. N Engl J Med 2000; 343: 1594–1602.
Targan SR, Hanauer SB, Van Devetler SJ, Mayer L, Present DH, Braakman T et al. The Crohn's disease cA2 study group. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor for Crohn's disease. N Engl J Med 1997; 337: 1029–1035.
Brandt J, Haibel H, Cornely D, Golder W, Gonzalez J, Redding J et al. Successful treatment of active ankylosing spondylitis with anti-tumor necrosis factor alpha monoclonal antibody infliximab. Arthritis Rheum 2000; 43: 1346–1352.
Joseph A, Raj D, Dua HS, Powell PT, Lanyon PC, Powell RJ . Infliximab in the treatment of refractory posterior uveitis. Ophthalmology 2003; 110: 1449–1453.
Nussenblatt RB, Palestine AG, Chan CC, Roberge F . Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology 1985; 92: 467–471.
Murphy CC, Ayliffe WH, Booth A, Makanjuola D, Andrews PA, Jayne D . Tumor necrosis factor alpha blockade with infliximab for refractory uveitis and scleritis. Ophthalmology 2004; 111: 352–356.
ten Tusscher MP, Jacobs PJ, Busch MJ, de Graaf L, Diemont WL . Bilateral anterior toxic optic neuropathy and the use of infliximab. BMJ 2003; 326: 579.
Foroozan R, Buono LM, Sergott RC, Savino PJ . Retrobulbar optic neuritis associated with infliximab. Arch Ophthalmol 2002; 120: 985–987.
Cursiefen C, Grunke M, Dechant C, Antoni C, Junemann A, Holbach LM . Multiple bilateral eyelid molluscum contagiosum lesions associated with TNFalpha-antibody and methotrexate therapy. Am J Ophthalmol 2002; 134: 270–271.
Baeten D, Kruithof E, Van den Bosch F, Van den Bossche N, Herssens A, Mielants H et al. Systematic safety follow up in a cohort of 107 patients with spondyloarthropathy treated with infliximab: a new perspective on the role of host defence in the pathogenesis of the disease? Ann Rheum Dis 2003; 62: 829–834.
Hage CA, Wood KL, Winer-Muram HT, Wilson SJ, Sarosi G, Knox KS . Pulmonary cryptococcosis after initiation of anti-tumor necrosis factor-alpha therapy. Chest 2003; 124: 2395–2397.
Tai TL, O'Rourke KP, McWeeney M, Burke CM, Sheehan K, Barry M . Pneumocystis carinii pneumonia following a second infusion of infliximab. Rheumatology 2002; 41: 951–952.
Alcain G, Andrade RJ, Queipo de Llano MP, Moreno MJ, Garcia-Cortes M, Franquelo E . Acute leukemia after infliximab therapy. Am J Gastroenterol 2003; 98: 2577.
Menon Y, Cucurull E, Espinoza LR . Pancytopenia in a patient with scleroderma treated with infliximab. Rheumatology 2003; 42: 1273–1274.
Baeten D, Kruithof E, Van den Bosch F, Van den Bossche N, Herssens A, Mielants H et al. Systematic safety follow up in a cohort of 107 patients with spondyloarthropathy treated with infliximab: a new perspective on the role of host defence in the pathogenesis of the disease? Ann Rheum Dis 2003; 62: 829–834.
Roberts SD, Wilkes DS, Burgett RA, Knox KS . Refractory sarcoidosis responding to infliximab. Chest 2003; 124: 2028–2031.
Author information
Authors and Affiliations
Corresponding author
Additional information
We transfer all copyright ownership to the Royal College of Ophthalmologists in the event this paper is published. None of the authors has a financial interest in any product mentioned.
Rights and permissions
About this article
Cite this article
Benitez-del-Castillo, J., Martinez-de-la-Casa, J., Pato-Cour, E. et al. Long-term treatment of refractory posterior uveitis with anti-TNFα (infliximab). Eye 19, 841–845 (2005). https://doi.org/10.1038/sj.eye.6701689
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6701689
Keywords
This article is cited by
-
The Use of Biologic Therapies in Uveitis
Clinical Reviews in Allergy & Immunology (2015)
-
Morbus Behçet – ophthalmologische und allgemeine Aspekte
Der Ophthalmologe (2013)