Abstract
Background:
The interval between successive births (birth interval) may affect breast cancer risk, whereas interval from last birth to cancer onset may modify its behaviour.
Methods:
The study cohort consisted of 29 488 Finnish grand multiparous (GM) women, including 628 women with breast cancer. Conditional logistic regression for case–control design nested within the cohort was used to estimate proportional hazards (referred as relative risks, RR). Age at first birth and parity were co-variables.
Results:
Short interval (<1 year) between first and second birth increased the risk of advanced ductal breast cancer at ages < 50 years (RR=5.29; 95% CI 2.00–14.0) as compared to interval 3+ years. The risk of advanced ductal cancer was also large (RR = 4.00; 95% CI 1.19–13.4) shortly (<3 years) after last birth as compared with the period 15+ years.
Conclusions:
Short birth interval-associated excess breast cancer risk may be related to stimulatory effects of female steroid hormones produced during two closely connected pregnancies, or defective breast maturation owing to failures in breastfeeding.
Similar content being viewed by others
Main
Findings in our previous study of grand multiparous women (GM, at least five births) indicated that birth interval (time between two successive births) might affect the risk of breast cancer (Hinkula et al, 2001). This finding and those of others (Albrektsen et al, 2005) prompted us to evaluate the impact of individual birth intervals on risk. Specific attention was paid to the interval between first and second births because the first pregnancy had a significant role in the maturation of the breast resistance against carcinogenic influences (Russo et al, 1994a, 2005). The length of birth interval depends also on the duration of breastfeeding, which is an important determinant of breast cancer risk (Collaborative Group of Hormonal in Breast Cancer, 2002; Ursin et al, 2005; Lord et al, 2008).
Pregnancies have dual effects on breast cancer: an immediate increase in the risk after childbirth is followed by a long-term protection (Woods et al, 1980; Kvåle and Heuch, 1987; Kelsey et al, 1993). In addition, cancers appearing within 2–6 years after birth are more frequently advanced than those in women having a long interval between birth and cancer onset (Kroman et al, 1997; Wohlfarth et al, 2001; Phillips et al, 2004; Rosenberg et al, 2004; Albrektsen et al, 2005, 2006).
This study population comprises of only GM women, most of whom belong to the religious minority, the Laestadian movement within the Lutheran church, which forbids the use of artificial contraception. Hence conception in this population takes place in physiological circumstances, thereby representing a specific advantage for our study. Here, we aimed to explore the impact of individual birth intervals on breast cancer risk at different stages. Another aim was to assess how the relative risk changes in relation to time since last birth in a Finnish cohort of GM women.
Materials and methods
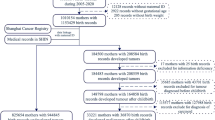
The data of the Finnish national Population Register comprised 29 488 women born 1935 or later and registered as having at least five biological children by the end of 1997. This database involves complete linkages between parents and the children born in October 1953 or later, but precluding our obtaining full parity history for women born before 1935. The exactly registered birthdays of each child form the basis for birth interval calculations.
Breast cancer cases diagnosed among the GM women between the fifth childbirth and 31 December 2006 were identified in an automatic record linkage with the files of the national population-based Finnish Cancer Registry, using personal identifiers as the key. There were 628 such breast cancers. The Finnish Cancer Registry files include clinical stage and cancer morphology. The registry receives cancer notifications from hospitals, pathological and cytological laboratories, and also from physicians outside hospitals; its coverage is almost 100% (Teppo et al, 1994).
Statistical methods
For each breast cancer case, 50 controls were randomly selected among the GM cohort members who were at risk for breast cancer at the time of cancer onset of the case and fulfilled the matching criteria; a tolerance of ±1 year was allowed on the date of birth. The proportional hazards method was applied to this case–control data. A woman was a non-case until she became a case, so controls for each case were selected among non-cases irrespective of whether they later became cases. The point estimates, hazard ratios were obtained in SAS 9.1 using the PHREG procedure with the option TIES=DISRETE, which requests the discrete logistic model. This method is same as the method of the conditional logistic regression analysis if the controls were selected among the individuals who were at risk of becoming cases. Proportional hazard were referred here as relative risks (RR). Possible interactions were evaluated using the TPHREG procedure, a test release of the PHREG procedure that incorporates the CLASS statement in SAS 9.1. No interactions were found between the study variables.
The RRs were also calculated for ductal and lobular, and for local and advanced (regional or distant metastases) breast cancers separately. Further stratification was based on the age at the diagnosis of cancer (<50 years and 50+ years). Pregnancy at the age of 50 years or later is extremely rare. The younger women represent thus the years of reproduction and the older ones that of ovarian quiescence. The distribution of patients into different subcategories is presented in Table 1.
The likelihood ratio test was used to evaluate statistical significance of the parameters of interest. The trend test was analysed using linear trend test for the classified variables. The lowest category had value 1 and the highest category had the same value as the number of classes of the respective variable. Each of the four intervals between first and fifth births was classified into three categories (<1, 1–2 and 3+ complete years). Parity (5, 6, 7, 8, 9 and 10+ children) and the age at first birth (<20, 20–24, 25–29, 30+ years) were added in each model. The time from the latest birth to the date of onset of cancer was stratified into four categories (<3, 3–6, 7–14, 15+ complete years).
Results
Overall, the RR did not differ significantly between different birth intervals, whereas short interval (<3 years) between last birth and cancer onset was associated with a significantly increased risk as compared to those with the interval of 15+ years (Table 2). Increase in the number of births and decline in age at first birth diminished the risk of breast cancer.
For cancers diagnosed before 50 years of age, the RR for short interval (<1 year) between first and second birth was increased twofold when compared with birth interval of 3+ years, and the interval from last birth to cancer onset among the cases was three times more often short (<3 years) than among the controls (Table 3). An increase in parity from 5 to 8+ nearly halved the RR in both age groups. In the group of 50+ year-old GM women, a decline in age at first birth decreased significantly the RR of breast cancer.
The most significant findings appeared in advanced ductal cancer diagnosed before age 50 years (Table 4). Short (<1 year) interval between first and second birth was associated with more than fivefold increased RR when compared with the 3+ years category. The RR of this cancer type appearing within 3 years after last birth was fourfold as compared with the 15+ years category. These factors operate separately; there was not a single breast cancer GM woman, with birth interval <1 year and interval from last birth to cancer <3 years. A long interval (15+ years) between last birth and cancer onset may protect against breast cancer at age 50+ years (Table 4).
Discussion
This study finds that among GM women, breast cancer is associated with birth interval. Most important was the new finding that short birth interval between first and second birth was significantly associated with increased risk of advanced ductal cancer in young GM mothers. The risk of ductal breast cancer in clinically advanced stage is also high during the 3 years following the last birth.
Previous studies on the risk factors in breast cancer have comprised predominantly nulliparous women and women with few children. We widened the perspective by evaluating this topic in a homogenous group of Finnish GM women, whose breast cancer risk is low, 45% having below the average incidence (Hinkula et al, 2001).
The registers used in this study, the National Population Register for births, and the Finnish Cancer Registry, are reliable and virtually complete (Teppo et al, 1994; Pukkala, 2009). Because the most important results in this study are from ages <50 years, the lack of information on postmenopausal hormone therapy or body weight does not weaken the validity of the findings.
The RR of advanced ductal breast cancer of GM women below 50 years of age was more than five times higher if the interval between first and second birth was <1 year (=pregnancy interval <3–4 months) as compared to 3+ years. The mechanism of this detrimental effect is unclear. The fact that it appeared only after the first pregnancy might reflect an association with breast maturation, because first pregnancy may have a central role in the differentiation of breast cells more resistant to carcinogenic influences (Lambe et al, 1994; Russo and Russo, 1994b; Chie et al, 2000; Russo et al, 2005; Wagner and Smith, 2005; Siwko et al, 2008). The suggestion that incomplete differentiation may predispose to breast cancer (Lagiou et al, 2003) has been supported by findings in experimental animal studies that an interaction of carcinogen with an undifferentiated breast epithelium is a prerequisite for carcinogenic initiation (Russo et al, 2001). It is possible that an insufficiently maturated breast of the young mother after her first childbirth – remains susceptible to carcinogenic effects, including hormonal influences.
Theories of hormonal mechanisms of breast cancer development are primarily based on exposure to excessive endogenous oestrogens (Colditz and Rosner, 2000; Yager and Davidson 2006). The influence of endogenous hormones on the risk of breast cancer of women may involve in addition to estrogens effects of progesterone, prolactin, testosterone and IGF-1 on terminal differentiation of mammary stem cells (Merrill et al, 2005). Estrogens and progesterone are mitogenic for epithelial breast cells, and may stimulate breast cell proliferation (Söderqvist, 1998). During pregnancy, progesterone induces lobular–alveolar development and differentiation for lactation (Lange et al, 1999), and estrogens regulate ductal growth (Mauvais-Jarvis et al, 1986). Older premenopausal and perimenopausal women have been claimed to be at increased risk (Alexander and Roberts, 1987), possibly implying an increased growth rate for present tumours during the menopause, perhaps due to the effects of oestrogen unopposed by progesterone.
Studies on the possible role of prolactin in breast cancer have yielded inconsistent results – protective or carcinogenic effects or no effect (Clevenger et al, 2003; Albrektsen et al, 2006). The most recent prospective study suggests that prolactin might increase the risk of breast cancer (Tworoger et al, 2007). During pregnancy, prolactin level rises strongly from the eighth week onwards reaching the peak concentration at term. Thereafter prolactin, the principal hormone for milk biosynthesis, remains elevated only in nursing mothers, in whom each breastfeeding induces a transient peak in its level. In non-lactating woman prolactin concentration declines rapidly, which allows early resumption of ovulatory menstrual cycle and early fecundation after the birth. The contraceptive effect of prolactin is not absolute; the efficacy weakens especially from the fourth postpartum month onward. Fecundation of lactating GM mother within 3–4 months after her first childbirth is thus possible but supposedly a rare phenomenon. In such a case, the joint actions of prolactin, (induced by suckling) and progesterone (from corpus luteum of the second pregnancy) would initiate, in the beginning of second pregnancy, abnormal cellular changes owing to the potential for breast cancer development. Alternatively, the long-term joint stimulatory actions of placental hormones (estrogens and progesterone) and prolactin during two closely consecutive pregnancies may serve as initiators for malignant transformation of epithelial breast cells.
In spite of our lack of breastfeeding data, we hypothesise that GM women, with increased breast cancer risk in association with a short interval between first and second birth, do not have breastfeed or have only for a very short time. Short-term breastfeeding, and especially no breastfeeding is associated with shorter birth interval (Rutstein 2005). An increase in breast cancer risk has been reported among premenopausal American women, who nursed their first (RR=1.37) or second (RR=1,44) child <1 month (Byers et al, 1985).
Because lactation participates in the differentiation of mammary epithelium in its terminal phase (Russo and Russo, 1994b; Russo et al, 2001, 2008), deficient breastfeeding of the first child might leave the breast cells susceptible to carcinogenic influences. This would be in accordance with the finding that lactation has a significant independent protective effect in breast cancer (Byers et al, 1985; Collaborative Group of Hormonal Factors in Breast Cancer, 2002; Ursin et al, 2005), particularly before menopause (Byers et al, 1985; Newcomb et al, 1994; Lord et al, 2008). Insufficient breastfeeding resulting in defective breast maturation might thus be the primary cause for breast cancer of young GM women in this specific subgroup.
The picture of breast cancer risk increase associated with birth intervals appeared to be more complicated than the previous one that long birth interval solely would affect the risk (Kvåle et al, 1987; Albrektsen et al, 2005, 2006). In the light of the present results the picture of adverse effects associated with birth intervals seems to be more complex than the view that only long birth intervals affect risk (Kvåle and Heuch, 1987; Albrektsen et al, 2005, 2006).
The RR of breast cancer of young GM women was highest shortly after their last birth, which is in accordance with earlier observations of mothers with few pregnancies (Kvåle and Heuch, 1987; Leon et al, 1995; Wohlfarth et al, 2001; Albrektsen et al, 2005, 2006). Transiently increased breast cancer risk after the last childbirth in this study showed no association with the number of pregnancies. The detrimental effect of short interval extended to GM women aged 50+ years. Transient increase risk after latest birth may even last for 15 years (Lambe et al, 1994; Liu et al, 2002).
Young age at first birth and increasing parity are established protective factors in breast cancer risk (La Vecchia et al, 1989; Layde et al, 1989; Lambe et al, 1996; Merrill et al, 2005). In this study, after adjusting for other study variables, age at first birth and parity were significant factors in GM women aged 50+ years. Among GM women <50 years, our previous study of the same cohort (Hinkula et al, 2001) that did not include birth intervals and age at last birth showed, that age at first birth – but not parity – is a significant risk factor. In contrast, in this study parity is a significant risk factor among younger GM women – but not age at first birth. Thus, the new age-related variables seem to weaken the age at first birth effect and strengthen the role of parity as an independent risk factor in this age period.
Elevated risk of advanced ductal breast cancer of young GM women with short interval between first and second birth might have an association with female steroid hormones and prolactin secreted during two closely consecutive pregnancies or with defective maturation of the breast owing to inappropriate nursing.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Albrektsen G, Heuch I, Hansen S, Kvåle G (2005) Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br J Cancer 92: 167–175
Albrektsen G, Heuch I, Thoresen S, Kvåle G (2006) Clinical stage of breast cancer by parity, age at birth and time since birth: A progressive effect of pregnancy hormones. Cancer Epidem Biomarker Prev 15: 65–69
Alexander FE, Roberts MM (1987) The menopause and breast cancer. J Epidemiol Commun Health 141: 94–100
Byers T, Graham S, Rzepka T, Marshall J (1985) Lactation and breast cancer. Evidence for a negative association in premenopausal women. Am J Epidemiol 121: 664–674
Chie WC, Hsieh C, Newcomb PA, Longnecker MP, Mittendorf R, Greenberg ER (2000) Age at any full-term pregnancy and breast cancer risk. Am J Epidemiol 151: 715–722
Clevenger CV, Furth PA, Hankinson SE, Schuler LA (2003) The role of prolactin in mammary carcinoma. Endocr Rev 24: 1–27
Colditz GA, Rosner B (2000) Cumulative risk of breast cancer to age 70 years according to risk factor status: Data from the nurses’ health study. Am J Epidemiol 152: 950–964
Collaborative Group of Hormonal Factors in Breast Cancer (2002) Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50 302 women with breast cancer and 96 973 women without this disease. Lancet 360: 187–195
Hinkula M, Pukkala E, Kyyrönen P, Kauppila A (2001) Grand multiparity and the risk of breast cancer: population-based study of Finland. Cancer Cause Control 12: 491–500
Kelsey J, Gammon MD, John EM (1993) Reproductive and hormonal risk factors; Reproductive factors and breast cancer. Epidemiol Rev 15: 36–46
Kroman N, Wohlfarth J, Andersen KW, Mouridsen HT, Westergaard T, Melbye M (1997) Time since childbirth and prognosis in primary breast cancer: population based study. Br Med J 315: 851–855
Kvåle G, Heuch I (1987) A prospective study of reproductive factors and breast cancer. Am J Epidemiol 126: 842–850
Lagiou A, Lagiou P, Vassilarou DS, Stoikidou M, Trichopoulos D (2003) Comparison of age at first full-term pregnancy between women with breast cancer and women with benign breast diseases. Int J Cancer 107: 817–821
Lambe M, Hsieh C-C, Chan H-W, Ekbom A, Trichopoulos D, Adami H-O (1996) Parity, age at first and last birth, and risk of breast cancer: a population-based study in Sweden. Breast Cancer Res Treat 38: 305–311
Lambe M, Hsieh C-C, Trichopoulos D, Ekbom A, Pavia M, Adami H-O (1994) Transient increase in the risk of breast cancer after giving birth. N Engl J Med 331: 5–9
Lange C, Richer J, Horwitz K (1999) Hypothesis: Progesterone primes breast cells for cross-talk with proliferative or antiproliferative signals. Mol Epidemiol 13: 1425–1429
La Vecchia C, Negri E, Boyle P (1989) Reproductive factors and breast cancer: an overview. Soz Praventiv Med 34: 101–107
Layde PM, Webster LA, Baughman AL (1989) The independent associations of parity, age at first full term pregnancy and duration of breastfeeding with the risk of breast cancer. J Clin Epidemiol 42: 963–973
Leon DA, Carpenter LM, Broeders MJM, Gunnarskog J, Murphy MFG (1995) Breast cancer in Swedish women before age 50: evidence of a dual effect of completed pregnancy. Cancer Cause Control 6: 283–291
Liu Q, Wuu J, Lambe M, Hsieh SF, Ekbom A, Hsieh CC (2002) Transient increase in breast cancer risk after giving birth: postpartum period with the highest risk. Cancer Cause Control 13: 299–305
Lord SJ, Bernstein L, Johnson KA, Malone KE, McDonald JA, Marchbanks PA, Simon MS, Strom ML, Press MF, Folger SG, Burkman RT, Deapen D, Spirtas R, Ursin G (2008) Breast cancer risk and hormone receptor status in older women by parity, age at first birth, and breastfeeding: a case-control study. Cancer Epidem Biomarker Prev 17: 1723–1730
Mauvais-Jarvis P, Kutten F, Gompel A (1986) Antiestrogen action of progesterone in breast tissue. Breast Cancer Res Treat 8: 179–187
Merrill RM, Fugal S, Novilla LB, Raphael MC (2005) Cancer risk associated with early and late maternal age at first birth. Gynecol Oncol 96: 583–593
Newcomb PA, Storer PE, Longnecker MP, Mittendorf R, Greenberg ER, Clapp RW, Burke KP, Willett WC, MacMahon B (1994) Lactation and a reduced risk of premenopausal breast cancer. N Engl J Med 330: 81–87
Phillips K-A, Milne RL, Friedlander ML, Jenkins MA, McCredie MRE, Giles GG (2004) Prognosís of premenopausal breast cancer and childbirth prior to diagnosis. J Clin Oncol 22: 699–705
Pukkala E (2009) Biobanks and registers in epidemioloical research of cancer. In: Methods in Biobanking, Dillner J (ed). Methods in Molecular Biology. Totowa: Humana Press
Rosenberg I, Thalib I, Adami HO, Hall P (2004) Childbirth and breast cancer prognosis. Int J Cancer 111: 772–776
Russo J, Balogh GA, Russo IH, the FCCC Hospital Network Participants (2008) Full term pregnancy induces a specific genomic signature in the human breast. Cancer Epidemiol Biomarker Prev 17: 51–66
Russo J, Hu YF, Silva IDCG, Russo IH (2001) Cancer risk related to mammary gland structure and development. Microscopy Res Tech 52: 204–223
Russo J, Moral R, Balogh GA, Mailo D, Russo IH (2005) The protective role of pregnancy in breast cancer. Breast Cancer Res 7: 131–142
Russo J, Romero AI, Russo IH (1994a) Architectural pattern of the normal and cancerous breast under the influence of parity. Cancer Epidemiol Biomarker Prev 3: 219–224
Russo J, Russo IH (1994b) Toward a physiological approach to breast cancer prevention. Cancer Epidemiol Biomarker Prev 3: 353–364
Rutstein SO (2005) Effects of birth interval on neonatal, infant and under-five years mortality and nutritional status in developing countries: evidence from the demographic and health surveys. Int J Gynecol Obst 89: S7–S24
Siwko SK, Dong J, Lewis MT, Liu H, Hilsenbeck SG, Li Y (2008) Evidence that an early pregnancy causes a persistent decrease in the number of functional mammary epithelial stem cells - implication for pregnancy-induced protection against breast cancer. Stem Cells 26: 3205–3209
Söderqvist G (1998) Effects of sex steroids on proliferation in normal mammary tissue. Ann Med 30: 511–524
Teppo L, Pukkala E, Lehtonen M (1994) Data quality and quality control of a population-based cancer registry. Experience in Finland. Acta Oncol 33: 365–369
Tworoger SS, Eliassen AH, Sluss P, Hankinson SE (2007) A prospective study of plasma prolactin concentrations and risk of premenopausal and postmenopausal breast cancer. J Clin Oncol 25: 1482–1488
Ursin G, Bernstein L, Lord SJ, Karim R, Deapen D, Press MF, Daling JR, Norman SA, Marchbanks PA, Folger SG, Simon MS, Stom BL, Burkman RT, Weiss LK, Spirtas R (2005) Reproductive factors and subtypes of breast cancer defined by hormone receptor and histology. Br J Cancer 93: 364–371
Wagner KU, Smith GH (2005) Pregnancy and stem cell behaviour. J Mammary Gland Biol Neopl 10: 25–36
Wohlfarth J, Andersen PK, Mouridsen HT, Melbye M (2001) Risk of late-stage breast cancer after a childbirth. Am J Epidemiol 153: 1079–1084
Woods KL, Smith SR, Morrison JM (1980) Parity and breast cancer risk; evidence of dual effect. Br Med J 281: 419–421
Yager JD, Davidson NE (2006) Estrogen carcinogenesis in breast cancer. N Engl J Med 354: 270–282
Acknowledgements
We thank Irma H Russo and Jose Russo for their valuable advise in the preparation of the article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Kauppila, A., Kyyrönen, P., Hinkula, M. et al. Birth intervals and breast cancer risk. Br J Cancer 101, 1213–1217 (2009). https://doi.org/10.1038/sj.bjc.6605300
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6605300
Keywords
This article is cited by
-
The effect of length of birth interval on the risk of breast cancer by subtype in grand multiparous women
BMC Cancer (2019)
-
Pregnancy-associated breast cancer: the risky status quo and new concepts of predictive medicine
EPMA Journal (2018)
-
Grand multiparity and reproductive cancer in the Jerusalem Perinatal Study Cohort
Cancer Causes & Control (2016)
-
Dual effect of short interval between first and second birth on ductal breast cancer risk in Finland
Cancer Causes & Control (2012)