Abstract
The intrauterine and early life environments have been linked to the etiology of breast cancer in prior studies. We prospectively examined whether maternal and newborn anthropometric factors as reported by the mother are related to an increased incidence of adult breast cancer in the daughter. We used data from 35,133 mother-daughter dyads of the Nurses’ Health Study (NHS) II and the Nurses’ Mothers’ Cohort Study. In 2001, living mothers of NHS II participants who were free of cancer completed a questionnaire on their pregnancy with the nurse and their nurse daughter’s early life experience. During 403,786 years of follow-up, 865 daughters developed incident cases of invasive breast cancer. Nurses with a birthweight of ≥4000 g had a 32% greater risk for breast cancer (multivariable-adjusted hazard ratio (HR) = 1.32, 95% confidence interval (CI) = 1.02–1.71, p-trend = 0.09) compared with those with birthweights of 3000–3499 g. Higher birth length tended to increase risk of premenopausal breast cancer (p for trend = 0.05). We further noted a modest U-shaped relation between maternal weight gain during pregnancy and premenopausal breast cancer incidence in the daughter. Fetal growth may contribute to shaping later life risk for breast cancer, especially prior to menopause.
Similar content being viewed by others
Introduction
Breast cancer is the most common malignancy among women worldwide, with an estimated 1.67 million new cancer cases diagnosed in 20121. Known and suspected risk factors for breast cancer operating during adult life leave substantial variation in rates of this tumor unexplained. Over the past couple of decades perinatal and early life characteristics have emerged as novel breast cancer risk factors with consistency across different study populations but with some international divergence.
In prior epidemiologic studies, high birthweight has been associated with greater risk of breast cancer2,3,4,5,6, although this is not true for all studies5,7. Intrauterine exposure to sex steroid hormones, growth hormone, insulin, insulin-like growth factors (IGF)-1, and IGF-2, and epigenetic variation are potential key pathways linking anthropometric variables in early life to adult breast cancer risk8. Because investigations from retrospective case-control studies relying on information collected from the cases and controls themselves leave room for differential misclassification, data from prospective longitudinal analyses are warranted to overcome this bias. In previous literature, fewer data have been available on maternal factors such as pre-pregnancy body mass index (BMI), and weight gain during pregnancy in relation to the risk of breast cancer in the daughter. However, with the global epidemic of obesity maternal body mass prior to and during pregnancy has substantially increased during the past couple of decades, with 39% of women with normal, 59% with overweight, and 56% with obese prepregnancy BMI exceeding the current U.S. Institute of Medicine (IOM) recommendations for gestational weight gain9.The implications of these rapidly changing intrauterine conditions for the daughters’ future breast cancer risk need to be investigated.
We therefore used data from the prospective Nurses’ Mothers’ Cohort Study to explore the relation of anthropometric variables in newborns including birthweight and birth length to their risk of developing breast cancer in adulthood; we also examined whether maternal pre-pregnancy BMI, height, and weight gain during pregnancy as reported by the mothers were associated with the incidence of breast cancer among daughters participating in the Nurses’ Health Study (NHS) II.
Methods
Study population
The NHS II cohort was established in 1989 when 116,680 nurses aged between 25 and 42 years from 14 U.S. states completed a mailed questionnaire on lifestyle factors and medical history10. Follow-up questionnaires were mailed to participating nurses every two years updating information on lifestyle factors and health. In 2001, 35 830 living mothers of NHSII who were cancer-free participants completed a questionnaire on their pregnancy with their nurse daughter and on her early life exposures forming the Nurses’ Mothers’ Cohort Study11. NHS II participants who were adopted or whose adoption status was unknown and those with missing information on the exposures of interest were excluded from the respective analyses.
The institutional review boards of the Brigham and Women’s Hospital and Harvard School of Public Health, Boston approved the study protocol. Response to the questionnaire was considered implied consent.
Exposure assessment
On the Mothers’ questionnaire, the nurses’ mothers were asked to report their height and weight before pregnancy in open-ended questions; this information was used to calculate pre-pregnancy BMI as kg/m2. Pre-pregnancy BMI was categorized following standard World Health Organization definitions of underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2). Mothers were also asked to report their gestational weight gain in pre-specified categories (<10, 10–14, 15–19, 20–29, 30–40, >40 lbs). Mothers further reported birthweight and birth length of their daughters as open-ended values. Birthweights recalled by the nurses’ mothers were highly concordant with birthweight information obtained from birth certificates (r = 0.85)12. Birthweight was coded using standard categories (<2500, 2500–2999, 3000–3499, 3500–3999, ≥4000 grams). Birth length was categorized in equally spaced intervals capturing the narrow distribution (<47, 47–49, 50–52, 53–55, >55 cm). Ponderal index was calculated from birth weight and length [Ponderal index = birthweight (grams) x 100/(birth length, cm)³] and categorized in quintiles.
Covariate information
Covariate information on tobacco use during pregnancy and gestational age was obtained from the Mothers’ questionnaire. Additional information on daughter’s characteristics, including race/ethnicity, family history of breast cancer, month and year of birth of the nurse, menopausal status, adult caloric intake, alcohol consumption, smoking, and BMI was available from the NHS II questionnaires. The derivation of menopausal status in this cohort has been previously described13.
Ascertainment of breast cancer
On follow-up questionnaires administered every two years, NHS participants were asked whether they had been diagnosed with breast cancer in the previous two years. To confirm the diagnosis, participants who reported a breast cancer diagnosis were asked for permission to review their relevant medical records and pathology reports. Due to the high accuracy of self-reported breast cancer diagnosis (>98%), nurse participants who reported a diagnosis of breast cancer but for whom medical records could not be obtained were classified as breast cancer cases.
Ascertainment of deaths
Deaths were identified by reports from the next of kin, postal authorities, or the National Death Index.
Statistical analysis
Cox proportional hazards regression models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of the incidence of breast cancer in the daughters across categories of infant birthweight, birth length, maternal pre-pregnancy BMI, height, and gestational weight gain. Participants contributed follow-up time from the return of the Nurses’ Mothers questionnaire in 2001 to the date of death, cancer diagnosis (except non-melanoma skin cancer), or end of follow-up on June 1, 2013. The basic model adjusted for the nurse’s age and follow-up cycle. Multivariable-adjusted models additionally included race (White, Non-White), family history of breast cancer (yes, no), maternal pre-pregnancy BMI (<18.5 kg/m2, 18.5–24.9 kg/m2, 25.0–30.0 kg/m2, >30 kg/m2), pregnancy weight gain (<10 lbs, 10–14 lbs, 15–19 lbs, 20–29, 30–40, +40 lbs), maternal smoking during pregnancy (no smoking, quit smoking during pregnancy, light smoker [<15 cigarettes/day], heavy smoker [≥15 cigarettes/day]), and gestational age (<38 weeks, 38–42 weeks, >42 weeks). In separate models, we included adult characteristics of the nurses including alcohol consumption, energy intake, smoking habits, and most recent pre-diagnostic (current) BMI during adulthood, all of which were updated during follow-up.
We further stratified our analytic models by menopausal status. To test for a linear trend across categories of exposures, we created a continuous variable using the midpoint of each category. Statistical tests were considered statistically significant at the 5% level. All statistical analyses were performed using the SAS statistical software version 9.4 (SAS Institute Inc, Cary, NC). The study has been conducted and reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines14.
Results
During 403,786 person-years of follow-up between 2001 and 2013, we documented 865 newly diagnosed invasive cases of breast cancer among NHS II participants. The distribution of perinatal and adult characteristics of the nurse daughters and of the maternal anthropometric pregnancy characteristics across categories of birthweight and pregnancy weight gain is shown in Tables 1 and 2, respectively. Birthweight was correlated with birth length and gestational age. Mothers of daughters with greater birthweight were more likely to have smoked less than those with a low birthweight, to have gained more weight during pregnancy, and had a slightly higher pre-pregnancy BMI. Mothers who smoked during pregnancy gained the least amount of weight on average.
Compared with daughters with birthweights of 3000–3499 g, those with a birthweight of ≥4000 g had a 32% greater incidence of adult breast cancer (multivariable-adjusted HR = 1.32, 95% CI = 1.01–1.71, p for trend: 0.10; Table 3). This association was restricted to premenopausal breast cancer (multivariable-adjusted HR = 1.32, 95% CI = 0.89–1.96), while no association was observed for postmenopausal breast cancer (multivariable-adjusted HR = 1.05, 95% CI = 0.69–1.59). We further noted a statistical trend of borderline significance for a greater risk of premenopausal breast cancer in daughters with increasing birth length (p for trend = 0.06; Table 4). Due to the high correlation between birthweight and birth length (0.47) we chose not to mutually adjust the analyses of these two variables. We also explored the role of Ponderal Index but did not observe any association with adult breast cancer incidence (Tables 3 and 4).
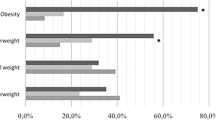
We did not note any important overall associations between maternal height, pre-pregnancy BMI, or maternal weight gain during pregnancy and daughter’s breast cancer risk (Tables 5 and 6). Daughters whose mothers had a pre-pregnancy BMI of ≥25 kg/m2 experienced a multivariable-adjusted HR of breast cancer of 0.98 (95% CI: 0.74–1.30) compared to those whose mothers were normal-weight (BMI 18.5–24.9 kg/m2; Table 5). We noted a modest U–shaped relation of maternal weight gain during pregnancy with the risk of premenopausal breast cancer in the daughters, but not for postmenopausal breast cancer (Table 6). For premenopausal breast cancer incidence in daughters comparing low (<15 lbs) versus normal maternal weight gain (20–29 lbs) during pregnancy the multivariable-adjusted HR was 1.23 (95% CI = 0.88–1.71) and comparing high (40+ lbs) versus normal weight gain the multivariable-adjusted HR was 1.17 (95% CI = 0.70–1.95). Additional adjustment for variables of the nurse’s adult life did not appreciably modify the results.
Discussion
In this study, a high birthweight was associated with a greater incidence of breast cancer later in life. Moreover, a positive trend with increasing birth length was noted for premenopausal breast cancer. We further observed a U-shaped relation between maternal weight gain during pregnancy and premenopausal breast cancer in daughters.
A positive association between high birthweight studies (from measurements made at birth by medical doctors, birth records/medical registers, maternal interviews, or adult self-report) and an increased risk of breast cancer in adulthood has been observed in most prior studies2,3,4,5,6,7,8 and confirms our earlier findings from the same cohort using self-reported birth weight4,8. The mechanisms underlying the association between a high birthweight and the diagnosis of breast cancer risk in later life is not entirely clear, but may be orchestrated by intrauterine exposures to growth hormones and epigenetic programming8,15. Birthweight is likely a marker for intrauterine levels of sex estrogen and progesterone, growth hormone, IGF-1, IGF-2, and insulin itself which may increase the number of susceptible stem cells in the mammary gland or enhance cell proliferation, thereby contributing to tumor development through accumulation of DNA mutations8. Since the epigenetic profile is established in utero, intrauterine stressors may affect the epigenetic pattern and therefor susceptibility to cancer16. The exposure to high levels of hormones and growth factors in utero may change the response of breast tissue to hormonal inputs later.
Our study revealed a statistically significant dose-response relation between birth length and premenopausal breast cancer. A previous meta-analysis suggested a positive association between birth length and later breast cancer in studies based on birth records, but not in studies based on parental recall or self-reports, likely reflecting the difficulty in precisely capturing birth length7. Unlike in our study, the birth length – breast cancer association was not modified by menopausal status in that meta-analysis7. The mechanisms underlying any association between birth length and subsequent breast cancer are likely similar to those connecting birthweight and breast cancer.
We observed a modest U-shaped relation between maternal weight gain during pregnancy and the daughter’s premenopausal breast cancer risk. A previous analysis by Sanderson et al.17 examining the relation between maternal weight gain during pregnancy reported by the mother and breast cancer risk in the daughters in two population-based case-control studies reported a higher breast cancer risk in daughters (HR = 1.50, 95% CI: 1.10–2.00) whose mothers gained 25–34 pounds compared to mothers who gained 15–24 pounds17. Notably, women whose mothers gained 35 pounds or more during pregnancy were not at an increased risk of breast cancer in that study17. We previously analyzed the relation between maternally reported pre-pregnancy BMI and gestational weight gain with breast cancer risk in adult daughters in a retrospective case-control study nested within the NHS I and NHS II and found no association18. Similar to the results in our current study, Sanderson et al.17 did not observe a link between maternal pre-pregnancy BMI and breast cancer risk in the daughters. However, both our study and that by Sanderson and colleagues were based on pregnancies several decades in the past, when in particular pre-pregnancy BMI, but also gestational weight gain recommendations were considerably lower than currently. Hence, these associations need to be further evaluated in more recent studies including a larger proportion of mothers with high pre-pregnancy BMI and maternal weight gain, reflecting current distributions.
Strengths of our study include its prospective design, the large number of study participants, and adjustment for a number of important potential perinatal confounding variables. Moreover, our study was based on a two-generation design providing the unique opportunity to use data collected directly from the mothers and combine them with data provided by the nurses.
A limitation of our study is that gestational and newborn characteristics had to be recalled by the mothers likely introducing random misclassification. However, the validity of birthweight reported by the mothers appears to be high when compared with birthweight recorded on state birth records (r = 0.85)12. Similarly, other studies have reported good agreement between pregnancy-related factors recorded during pregnancy and maternal recall19,20,21. The nurses’ adult life variables did not appreciably affect associations observed. However, we cannot rule out unmeasured confounding or residual confounding through covariates measured with error. Finally, with mothers being born between 1921 and 1964, we had limited power to examine substantial pregnancy weight gains and high values of pre-pregnancy BMI which have become more prevalent in recent years.
In summary, a high birthweight and possibly a high birth length may predict an increased risk of breast cancer in later life, particularly of premenopausal breast cancer. Whether maternal factors during pregnancy and other infant factors are related to adult breast cancer risk requires further clarification in other prospective studies with good exposure validity.
Data availability
All relevant data are provided within the manuscript or are available from the corresponding author upon request.
References
Ferlay, J. et al. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC cancer base no. 11 [Internet]. International Agency for Research on Cancer, Lyon (2014).
Troisi, R. et al. Perinatal characteristics and breast cancer risk in daughters: a Scandinavian population-based study. J. Dev. Orig. Health Dis. 4, 35–41 (2013).
Spracklen, C. N., Ryckman, K. K., Harland, K. & Saftlas, A. F. Effects of smoking and preeclampsia on birth weight for gestational age. J. Matern. Fetal Neonatal Med. 28, 679–84 (2015).
Xue, F., Rosner, B., Eliassen, H. & Michels, K. B. Body fatness throughout the life course and the incidence of premenopausal breast cancer. Int. J. Epidemiol. 45, 1103–1112 (2016).
Kar, S. P. et al. The association between weight at birth and breast cancer risk revisited using Mendelian randomisation. Eur. J. Epidemiol. 34, 591–600 (2019).
Barber, L. E., Bertrand, K. A., Rosenberg, L., Battaglia, T. A. & Palmer, J. R. Pre- and perinatal factors and incidence of breast cancer in the Black Women’s Health Study. Cancer Causes Control. 30, 87–95 (2019).
Silva Idos, S., De Stavola, B. & McCormack, V. Collaborative Group on Pre-Natal Risk, F. & Subsequent Risk of Breast, C. Birth size and breast cancer risk: re-analysis of individual participant data from 32 studies. Plos Med. 5, e193 (2008).
Michels, K. B. & Xue, F. Role of birthweight in the etiology of breast cancer. Int. J. Cancer J. Int. Cancer 119, 2007–2025 (2006).
Dalenius, K., Brindley, P. L., Smith, B. L., Reinold, C. M. & Grummer-Strawn, L. Pregnancy nutrition surveillance: 2010 report (2012).
Rich-Edwards, J. W. et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am. J. Obstet. Gynecol. 171, 171–177 (1994).
Michels, K. B. et al. A longitudinal study of infant feeding and obesity throughout life course. Int. J. Obes. 2005 31, 1078–1085 (2007).
Troy, L. M. et al. Self-reported birthweight and history of having been breastfed among younger women: an assessment of validity. Int. J. Epidemiol. 25, 122–127 (1996).
Rosner, B. & Colditz, G. A. Age at menopause: imputing age at menopause for women with a hysterectomy with application to risk of postmenopausal breast cancer. Ann. Epidemiol. 21, 450–460 (2011).
von Elm, E. et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335, 806–8 (2007).
Michels, K. B. Early life predictors of chronic disease. J. Womens Health 2002 12, 157–161 (2003).
Mansell, T. & Saffery, R. The end of the beginning: epigenetic variation in utero as a mediator of later human health and disease. Epigenomics 9, 217–221 (2017).
Sanderson, M. et al. Maternal factors and breast cancer risk among young women. Paediatr. Perinat. Epidemiol. 12, 397–407 (1998).
Wilson, K. M., Willett, W. C. & Michels, K. B. Mothers’ pre-pregnancy BMI and weight gain during pregnancy and risk of breast cancer in daughters. Breast Cancer Res. Treat. 130, 273–279 (2011).
Skulstad, S. M. et al. Validation of maternal reported pregnancy and birth characteristics against the Medical Birth Registry of Norway. Plos One 12, e0181794 (2017).
Liu, J., Tuvblad, C., Li, L., Raine, A. & Baker, L. A. Medical record validation of maternal recall of pregnancy and birth events from a twin cohort. Twin Res. Hum. Genet. 16, 845–60 (2013).
Rice, F. et al. Agreement between maternal report and antenatal records for a range of pre and peri-natal factors: the influence of maternal and child characteristics. Early Hum. Dev. 83, 497–504 (2007).
Acknowledgements
We thank the participants and staff of the Nurses’ Health Study II cohorts, for their valuable contributions. We thank the following state cancer registries for their help: A.L., A.Z., A.R., C.A., C.O., C.T., D.E., F.L., G.A., I.D., I.L., I.N., I.A., K.Y., L.A., M.E., M.D., M.A., M.I., N.E., N.H., N.J., N.Y., N.C., N.D., O.H., O.K., O.R., P.A., R.I., S.C., T.N., T.X., V.A., W.A. and W.Y. The Nurses’ Mothers’ Cohort Study was funded by the Intramural Program of the National Cancer Institute (Research Contract N02-RC-17027). The Nurses’ Health Study II is supported by Public Health Service grant CA50385 and by grant U01CA176726 from the National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services. The authors assume full responsibility for analyses and interpretation of the data.
Author information
Authors and Affiliations
Contributions
D.S. and K.M. designed the study; K.M. and W.W. collected the data; D.S. and M.D. analyzed the data; D.S. wrote the initial draft; all authors reviewed and revised the draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schmid, D., Willett, W.C., Ding, M. et al. Maternal and Infant Anthropometric Characteristics and Breast Cancer Incidence in the Daughter. Sci Rep 10, 2550 (2020). https://doi.org/10.1038/s41598-020-59527-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-59527-w
This article is cited by
-
Association of birth weight with cancer risk: a dose–response meta-analysis and Mendelian randomization study
Journal of Cancer Research and Clinical Oncology (2023)
-
Perinatal factors, female breast cancer, and associated risk factors in Puerto Rico: evidence from the Atabey epidemiology of breast cancer study
Cancer Causes & Control (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.